Chapter 5 Acid-Base Equilibria
Key concepts:
- Acids react with water to produce hydronium and conjugate base
- Bases react with water to produce hydroxide and conjugate acid
- Acids and bases react with each other (neutralization reaction)
Note: Equilibrium values are given at 25 °C in water unless otherwise stated.
We define the following notation for acids and bases
- Acid: HA, HB+
- Base: B, A–
There are different definitions for acids and bases.
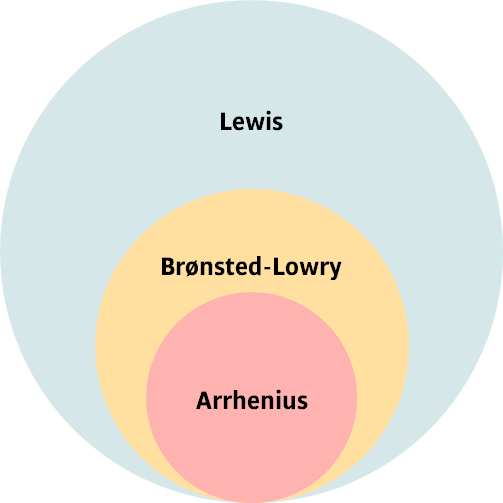
An Arrhenius acid is is a substance that dissociates in water to produce H+ ions.
\[\color{green}{\mathrm{HCl}}(aq) + \mathrm{H_2O}(l) \rightleftharpoons \mathrm{H_3O^+}(aq) + \color{red}{\mathrm{Cl^-}}(aq)\]
or generally
\[\color{green}{\mathrm{HA}}(aq) + \mathrm{H_2O}(l) \rightleftharpoons \mathrm{H_3O^+}(aq) + \color{red}{\mathrm{A^-}}(aq)\]
An Arrhenius base is is a substance that dissociates in water to produce OH– ions.
\[\color{red}{\mathrm{NH_3}}(aq) + \mathrm{H_2O}(l) \rightleftharpoons \mathrm{OH^-} + \color{green}{\mathrm{NH_4^+}}(aq)\]
or generally
\[\color{red}{\mathrm{B}}(aq) + \mathrm{H_2O}(l) \rightleftharpoons \mathrm{OH^-}(aq) + \color{green}{\mathrm{HB^+}}(aq)\]
A Brønsted-Lowry acid is a substance that donates a proton (i.e. is a proton donor).
\[\color{green}{\mathrm{HCl}}(aq) + \mathrm{H_2O}(l) \rightleftharpoons \mathrm{H_3O^+}(aq) + \color{red}{\mathrm{Cl^-}}(aq)\]
Here, HCl, an acid, donates its proton to water. A general scheme can be written as
\[\color{green}{\mathrm{HA}}(aq) + \mathrm{H_2O}(l) \rightleftharpoons \mathrm{H_3O^+}(aq) + \color{red}{\mathrm{A^-}}(aq)\]
A Brønsted-Lowry base is a substance that accepts a proton (i.e. is a proton acceptor).
\[\color{red}{\mathrm{NH_3}}(aq) + \mathrm{H_2O}(l) \rightleftharpoons \mathrm{OH^-}(aq) + \color{green}{\mathrm{NH_4^+}}(aq)\]
Here, NH3, an base, accepts a proton from water. A general scheme can be written as
\[\color{red}{\mathrm{B}}(aq) + \mathrm{H_2O}(l) \rightleftharpoons \mathrm{OH^-}(aq) + \color{green}{\mathrm{HB^+}}(aq)\] or
\[\color{red}{\mathrm{A^-}}(aq) + \mathrm{H_2O}(l) \rightleftharpoons \mathrm{OH^-}(aq) + \color{green}{\mathrm{HA}}(aq)\]
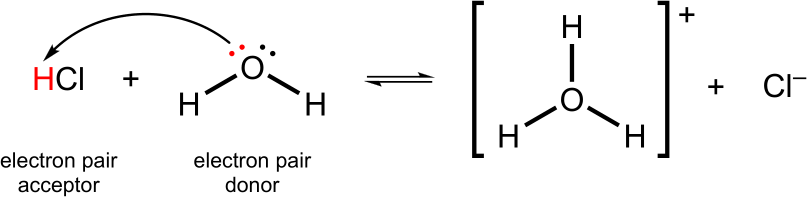
A Lewis acid is a substance that accepts a pair of electrons (i.e. a lone-pair acceptor).
\[\color{green}{\mathrm{HCl}}(aq) + \mathrm{H_2O}(l) \rightleftharpoons \mathrm{H_3O^+}(aq) + \color{red}{\mathrm{Cl^-}}(aq)\]
Here, HCl dissociates into H+ and Cl–. The oxygen on water donates a lone pair of electrons to the free proton and forms a bond to give the hydronium ion, H3O+.
Lewis Acid Example
Water behaves as a Lewis base by donating an electron pair to HCl. HCl behaves as a Lewis acid by accepting the lone electron pair from water.
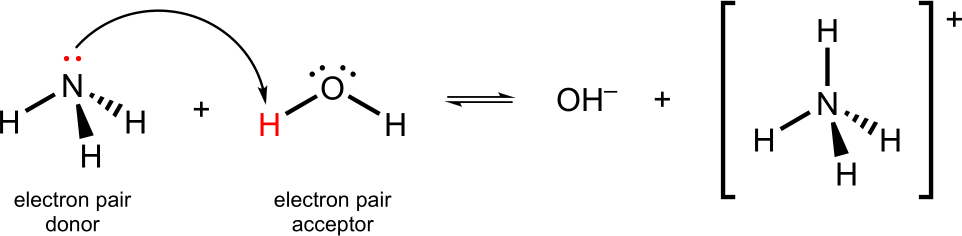
A Lewis base is a substance that donates a lone pair of electrons (i.e. lone-pair donor).
\[\color{red}{\mathrm{NH_3}}(aq) + \mathrm{H_2O}(l) \rightleftharpoons \mathrm{OH^–}(aq) + \color{green}{\mathrm{NH_4^+}}(aq)\]
Here, the lone pair on the nitrogen in ammonia, NH3, is donated to a proton on water forming a bond to give NH4+.
Lewis Base Example
Water behaves as a Lewis acid by accepting the lone electron pair from ammonia. Ammonia behaves as a Lewis base by donating an electron pair to water.
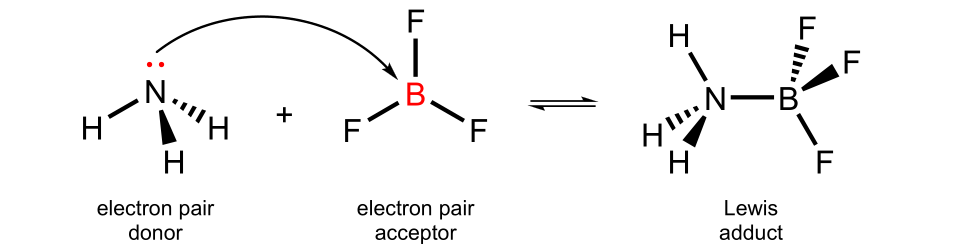
In this example, ammonia forms a Lewis adduct with boron triflouride. No proton is transferred in this addition reaction. The N–B covalent bond is a dative bond, one that forms from an electron pair from one species.
Practice
Determine the type of acid (or base) that HCN is behaving as. Which definition(s) does it comply with?
\[\mathrm{HCN}(aq) + \mathrm{H_2O}(l) \rightleftharpoons \mathrm{H_3O^+}(aq) + \mathrm{CN^-}(aq)\]
Solution
- Arrhenius acid - HCN produces hydronium when dissolved in water
- Brønsted-Lowry acid - HCN donates a proton to water
- Lewis acid - HCN accepts a lone electron pair from water
The word conjugate means “joined together” (e.g. coupled) especially “in pairs”. In acid/base chemistry, a conjugate is a pairing of reactant and product. For example, a deprotonated acid, A–), is the conjugate base of the starting acid, HA.
\[\begin{alignat*}{3} \color{green}{\mathrm{HA}} ~~~ &\rightleftharpoons ~~~~~~ \mathrm{H^+} ~~ &&+ ~~~~~~~~~~~\color{red}{\mathrm{A^-}}\\[1.5ex] \mathrm{acid} ~~~ &\rightleftharpoons ~~ \mathrm{proton}~~ &&+ ~~\mathrm{conjugate~base} \end{alignat*}\]
An acid can also be generalized as HB+.
\[\begin{alignat*}{3} \color{green}{\mathrm{HB^+}} ~~~ &\rightleftharpoons ~~~~~~ \mathrm{H^+} ~~ &&+ ~~~~~~~~~~~ \color{red}{\mathrm{B}}\\[1.5ex] \mathrm{acid} ~~~ &\rightleftharpoons ~~ \mathrm{proton} ~~ &&+ ~~ \mathrm{conjugate~base} \end{alignat*}\]
To determine the conjugate base of any acid, simply deprotonate the acid (remove an H+). The resulting species is the conjugate base.
A conjugate acid is the opposite of a conjugate base. A conjugate acid can be generally written as HB+ or HA.
\[\begin{alignat*}{3} \color{red}{\mathrm{B}}~~ &~~~+~~~~~~ \mathrm{H^+} ~~~ &&\rightleftharpoons ~~~~~~~~~~~ \color{green}{\mathrm{HB^+}} \\[1.5ex] \mathrm{base} &~~~+~~ \mathrm{proton} ~~~ &&\rightleftharpoons ~~ \mathrm{conjugate~acid} \end{alignat*}\]
\[\begin{alignat*}{3} \color{red}{\mathrm{A^-}} &~~~+~~~~~~ \mathrm{H^+} ~~~ &&\rightleftharpoons ~~~~~~~~~~~ \color{green}{\mathrm{HA}} \\[1.5ex] \mathrm{base} &~~~+~~ \mathrm{proton} ~~~ &&\rightleftharpoons ~~ \mathrm{conjugate~acid} \end{alignat*}\]
To determine the conjugate acid of any base, simply protonate the base (add an H+). The resulting species is the conjugate acid.
Practice
Identify the conjugate (and type) to each of the following:
- HNO3 (acid)
- NH4+ (acid)
- OH– (base)
Solution
- NO3– ; conjugate base
- NH3 ; conjugate base
- H2O ; conjugate acid
An amphoteric substance is one that can behave both as an acid and a base depending on the environment it is in. An amphiprotic substance is a type of amphoteric substance and is one that can accept or donate a proton, H+.
One fundamental example is water, an amphiprotic substance. When an acid is added to water, water accepts a proton (and behaves as a base). When a base is added to water, water donates a proton (and behaves as an acid).
Aluminum hydroxide is an example of an amphoteric substance. When reacting with an acid, such as HCl, Al(OH)3 acts as a Lewis base.
\[\mathrm{Al(OH)_3}(aq) + \mathrm{3HCl}(aq) \rightleftharpoons \mathrm{AlCl_3}(aq) + \mathrm{3H_2O}(l)\]
Al(OH)3 behaves as a Lewis acid when reacting with NaOH (molecular equation given below).
\[\mathrm{Al(OH)_3}(aq) + \mathrm{NaOH}(aq) \rightleftharpoons \mathrm{Na[Al(OH)_4]}(aq)\]
We could write the above reaction in its net ionic form as
\[\mathrm{Al(OH)_3}(aq) + \mathrm{OH^-}(aq) \rightleftharpoons \mathrm{Al(OH)_4^-}(aq)\]