2.9 Solubility of Gases
Let us now examine the aqueous solubility of gases. The presented mass % data is from the CRC Handbook of Chemistry and Physics 97th ed. (p. 5-314)4.
The solubility of many gases are quite small and decrease with increasing temperature.
Figure 2.13: Aqueous solubility of some gases at various temperatures.
Notice the large solubility of carbon dioxide in water relative to other gases. Earth’s oceans absorb about 30% of the CO2 that is released into the atmosphere. Carbon dioxide can react with water to produce carbonic acid via the following process
\[\mathrm{CO_2}(aq) + \mathrm{H_2O}(l) \longrightarrow \mathrm{H_2CO_3}(aq)\] This leads to ocean acidification. Ocean water is slightly basic but has become more acidic (ΔpH = –0.11) since the industrial revolution.
2.9.1 Henry’s Law
Though increasing temperature lowers the solubility of a gas, increasing pressure will raise the solubility of a gas. Henry’s Law states that the concentration of a gas is proportional to the partial pressure of the gas above the liquid.
Aqueous solubility of gases increase with increasing pressure.
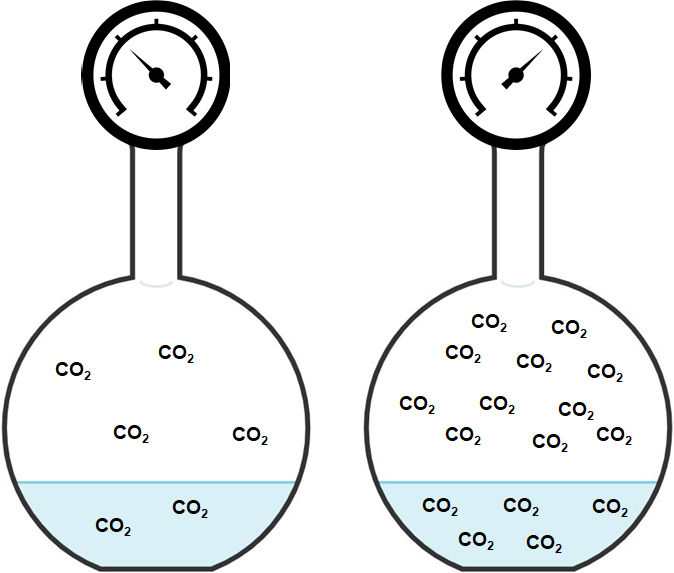
The ratio of gas concentration (Cg) vs pressure (Pg) is constant (called Henry’s constant; kH) and is unique to the identity of the gas. Across modest concentrations, the relationship is linear and can be described by the following equation
\[C_{\mathrm{g}} = k_{\mathrm{H}} P_{\mathrm{g}}\]
For example, a Henry’s constant for carbon dioxide is reported to be 3.4 × 10–4 mol m–3 Pa–1 at room temperature6. Let us convert the concentration (mol m–3) to molarity using the following known relationships:
- 1 m3 = 1000 L
- 1 atm = 101325 Pa
\[\begin{align*} k_{\mathrm{H}} &= \dfrac{3.4\times 10^{-4}~\mathrm{mol}}{\mathrm{m^3~Pa}} \left ( \dfrac{1~\mathrm{m^3}}{10^3~\mathrm{L}} \right ) \left ( \dfrac{101,325~\mathrm{Pa}}{1~\mathrm{atm}} \right ) \\[1.5ex] &= 0.03445~\mathrm{mol~L^{-1}~atm^{-1}} \end{align*}\]
Therefore, for every 1 atm of pressure, the concentration of carbon dioxide in water increases by 0.03445 mol L–1. We can plot the predicted solubility of CO2 vs a small range of pressures.
Figure 2.14: Aqueous solubility of carbon dioxide at various pressures.