3.8 Reaction Mechanisms
Chemical reactions can be categorized as complex reactions or elementary reactions. Consider the reaction we have been working with.
\[2\mathrm{NO_2}(g) \longrightarrow \mathrm{2NO}(g) + \mathrm{O_2}(g)\]
This reaction looks seemingly simple. We might look at this and interpret this reaction as two NO2 molecules colliding to undergo a chemical change to make products. However, this reaction is actually a multi-step reaction.
First Proposed Mechanism, slow second-step
One proposed mechanism is the following two-step mechanism.
\[\begin{align*} 2\mathrm{NO_2}(g) &\underset{k_{-1}}{\stackrel{k_1}{\rightleftharpoons}} \mathrm{(NO_2)_2}(g) &&\mathrm{(fast)} \\[1.5ex] \mathrm{(NO_2)_2}(g) &\overset{k_2}\longrightarrow \mathrm{2NO}(g) + \mathrm{O_2}(g) &&\mathrm{(slow)} \end{align*}\]
Adding both elementary steps together sums to the overall reaction.
The proposed set of steps for the overall reaction is called a reaction mechanism. The molecularity of the first step is bimolecular (involving two reactant particles) whereas the molecularity of the second step is unimolecular (involving one reactant particle). The first step produces an intermediate of reaction, a particle that a reaction produces and then later consumes. Here, the intermediate is (NO2)2 (e.g. the NO2 dimer) and this step is fast. The second step is the slower of the two. Because the second step is the slowest step in the mechanism, it is referred to as the rate determining step and this is the step that governs the overall rate of the reaction.
Elementary reactions involving the collision of 3 particles are called termolecular but these types of reactions are very rare.
We can write a rate law for each elementary step.
\[\begin{align*} \mathrm{rate}_1 &= k_1[\mathrm{NO_2}]^2 \\[1.5ex] \mathrm{rate}_2 &= k_2[(\mathrm{NO_2})_2] \end{align*}\]
where rate1 > rate2. The first step is second-order and the second step is first-order.
NOTE: Here that the exponents in the written rate laws come from the stoichiometric coefficients of the balanced elementary chemical equations. We can only do this with elementary reactions. With complex reactions, we must use the method of initial rates to determine the orders.
Let us reiterate the fact that the rate of this reaction is dictated by the slowest step of the reaction. Therefore, the rate law might be predicted to be
\[\mathrm{rate}_2 = k_2[(\mathrm{NO_2})_2] \qquad \longleftarrow \mathrm{this~is~wrong!}\] The (NO2)2 complex is an intermediate of reaction and not a reactant. This cannot be the appropriate rate law for the overall reaction since rate laws are expressed in terms of the reactants! What do we do here?
Take a look at that first step. It is a reversible step (see the double harpoons)? The first step is fast and when two NO2 molecules reacts to produce (NO2)2, we will see a rapid build up in intermediate. The second step of the reaction (the rate determining step) is chugging along slowly, consuming intermediate but it will not be able to keep up with how fast step 1 is producing it. The intermediate is hanging around in large quantities and is highly reactive. It readily dissociates into 2NO2.
Here’s the key. The first step will reach equilibrium. The rate at which (NO2)2 is produced will eventually equal the rate at which it is consumed (whether in step 2 or by dissociating and returning to 2NO2). We say that the rate of the forward reaction is equal to the rate of the reverse reaction.
Let us right out the rate laws for the forward and reverse reaction of step 1.
\[\begin{align*} \mathrm{rate}_{1,\mathrm{f}} &= k_1[\mathrm{NO_2}]^2 \\[1.5ex] \mathrm{rate}_{1,\mathrm{r}} &= k_{-1}[\mathrm{(NO_2)_2}] \end{align*}\]
At equilibrium, these two rates are equal. Follow the math here.
\[\begin{align*} \mathrm{rate}_{1,\mathrm{f}} &= \mathrm{rate}_{1,\mathrm{r}} \quad \therefore \\[1.5ex] k_1[\mathrm{NO_2}]^2 &= k_{-1}[\mathrm{(NO_2)_2}] \end{align*}\]
Remember how we said that the rate law for the overall reaction should only be expressed in terms of reactants? We can eliminate the intermediate term from the rate law by simply expressing it in terms of NO2. Watch this.
\[\begin{align*} k_1[\mathrm{NO_2}]^2 &= k_{-1}[\mathrm{(NO_2)_2}] \\[1.5ex] [\mathrm{(NO_2)_2}] &= \dfrac{k_1}{k_{-1}}[\mathrm{NO_2}]^2 \end{align*}\]
Here we “solved” for (NO2)2. Why? Take the right side of the expression and plug it into our “good guess but ultimately wrong” rate law!
\[\begin{align*} \mathrm{rate} &= k_2[(\mathrm{NO_2})_2] \qquad &&\longleftarrow \mathrm{this~is~wrong!} \\[1.5ex] &= k_2\dfrac{k_1}{k_{-1}}[\mathrm{NO_2}]^2 \\[1.5ex] &= k_2K_1[\mathrm{NO_2}]^2 \\[1.5ex] \mathrm{rate} &= k[\mathrm{NO_2}]^2 &&\longleftarrow \mathrm{this~is~right!} \end{align*}\]
The k1/k–1 term becomes K1, an equilibrium constant for the fast, reversible step and K1 × k2 becomes another rate constant. We will see equilibrium constants in the next chapter.
We can draw a reaction diagram for this reaction. A reaction diagram places the structures (reactants, products, intermediates, and transition states) on an energy diagram in the order that the reaction takes place.
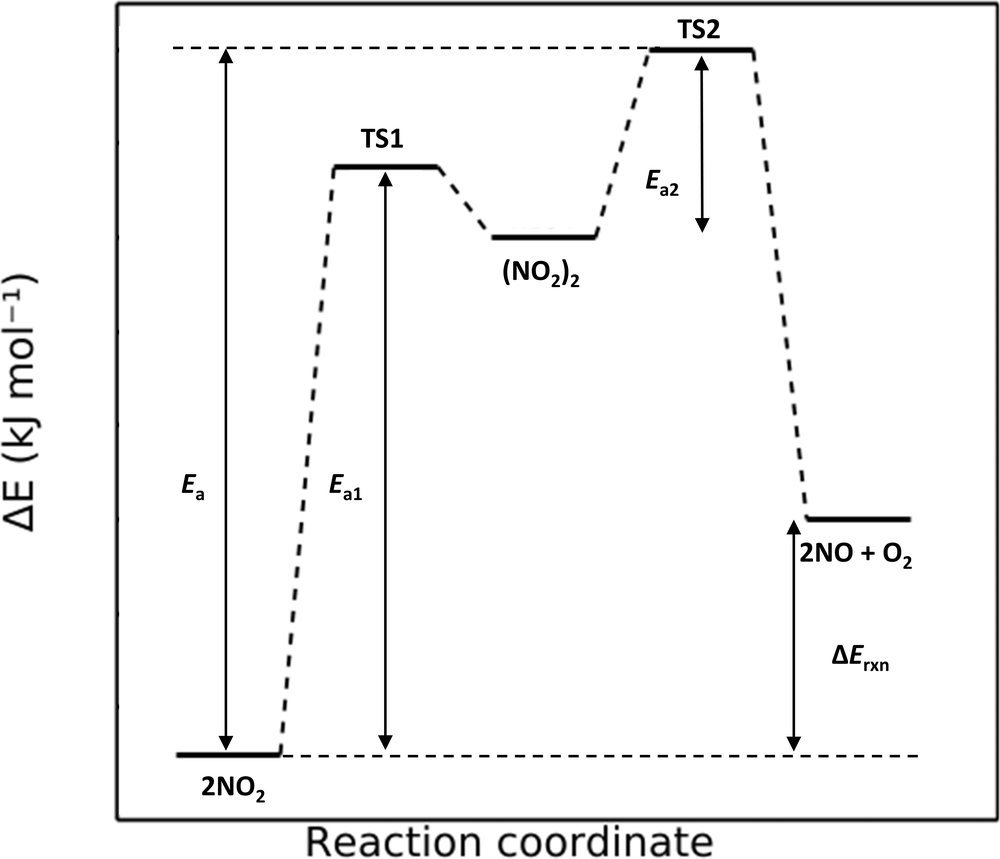
Figure 3.25: Reaction pathway
Let us break down this diagram. First, there are five stationary points shown (reactants, two transition-states, an intermediate, and products) and their corresponding relative energies. Transition-states are generally higher in energy than the points they connect. The reaction is exothermic since the products of reaction is lower in energy than the reactants.
The plot indicates a two-step reaction. The first step shows reactants proceeding through a transition state (TS2) and onward to the intermediate of reaction. The second step shows the intermediate proceeding to products through TS2. While each individual step has an activation energy, the activation energy of the overall reaction is given by step 2 as this is the largest energy change between reactants and the highest energy stationary point on the diagram. Step 1 is fast whereas step two is slow. This means that step 2 is the rate determining step (it contains the highest energy barrier in the entire reaction). If the energies were enthalpies, the reaction is endothermic (the products are higher in energy than the reactants).
Second Proposed Mechanism, slow first-step
The second proposed mechanism is the following two-step mechanism.
\[\begin{align*} 2\mathrm{NO_2}(g) &\overset{k_1}\longrightarrow \mathrm{NO_3}(g) + \mathrm{NO}(g) &&\mathrm{(slow)} \\[1.5ex] \mathrm{NO_3}(g) + \mathrm{NO}(g) &\underset{k_{-2}}{\stackrel{k_2}{\rightleftharpoons}} \mathrm{2NO}(g) + \mathrm{O_2}(g) &&\mathrm{(fast)} \end{align*}\]
Here, the first step is slow while the second step is fast. The predicted rate law (which should depend on the slowest step of the reaction) is predicted to be
\[\mathrm{rate} = k[\mathrm{NO_2}]^2\] which matches our predicted rate law for the first proposed mechanism! In fact, both mechanisms are plausible. The rate laws between the two are in agreement, both reaction mechanisms are bimolecular, and both reaction diagrams indicate an endothermic reaction!
Here is the reaction diagram for this mechanism.
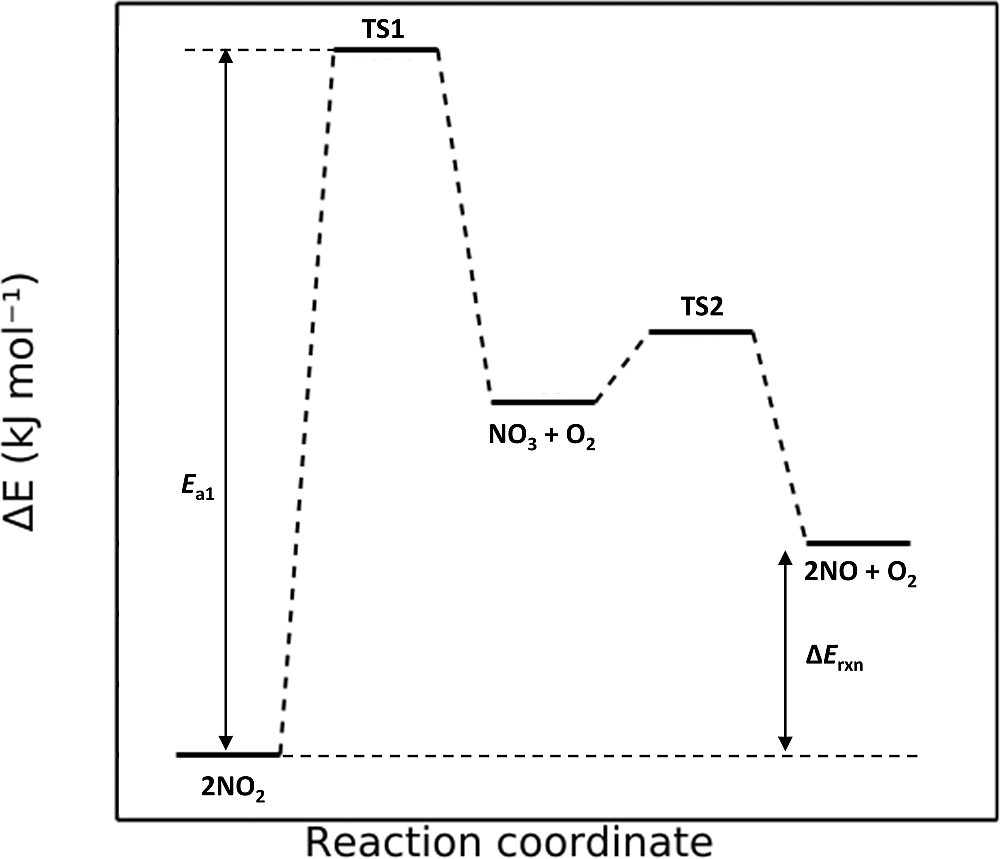
Figure 3.26: Reaction pathway
Practice
Determine the following for the given reaction diagram:
- Endothermic or Exothermic
- Number of transition states
- Number of intermediates
- Number of steps
- Slowest step
Solution
- Endothermic
- Transition states: 2
- Intermediates: 1
- Steps: 2
- Slowest step: Step 1
Practice
Determine the following for the given reaction diagram:
- Endothermic or Exothermic
- Number of transition states
- Number of intermediates
- Number of steps
- Slowest step
Solution
- Exothermic
- Transition states: 4
- Intermediates: 3
- Steps: 4
- Slowest step: Step 4