5.8 Salt Hydrolysis
Hydrolysis is the reaction of a compound with water where water breaks one or more chemical bonds in the reacting compound. Salt hydrolysis is, therefore, the reaction of salt with water, where one or more bonds is broken in the salt. A familiar example of this is the dissolution of sodium chloride in water where the ionic bond is broken.
\[\mathrm{NaCl}(s) \overset{\mathrm{H_2O}}\longrightarrow \mathrm{Na^+}(aq) + \mathrm{Cl^-}(aq)\]
Some salts, when dissolved in water, may affect the pH of the solution. Acidic salts lower the pH of solution, basic salts raise the pH of solution, and neutral salts have no effect on the pH of solution.
5.8.1 Acidic Salts
An acidic salt is any salt that lowers the pH of a solution. Consider ammonium chloride, NH4Cl. The hydrolysis of this salt is given as
\[\mathrm{NH_4Cl}(s) \overset{\mathrm{H_2O}}\longrightarrow \mathrm{NH_4^+}(aq) + \mathrm{Cl^-}(aq)\] Let us consider the reaction of each product of this reaction (i.e. the cation and anion of the salt) with water.
Cation
The ammonium ion, NH4+, is a conjugate acid to a weak base. It is the conjugate acid to ammonia, NH3. We can easily recognize this fact by simply deprotonating ammonium (removing a proton) which leaves us with the weak base, NH3! Next, recall that weak bases give rise to stronger conjugate acids. Since this is the case here, the acid (NH4+) will behave as such and react with water to produce hydronium!
\[\mathrm{NH_4^+}(aq) + \mathrm{H_2O}(l) \rightleftharpoons \mathrm{H_3O^+}(aq) + \mathrm{NH_3}(aq)\]
This is further understood by applying the fact that weak bases can exist in water. Here, ammonia, NH3 is being produced and rightfully so. When this reaction occurs, the pH of the solution is lowered due to the production of H3O+.
Anion
Next, consider the anion of the salt. Will the chloride ion react with water to produce HCl?
\[\mathrm{Cl^-}(aq) + \mathrm{H_2O}(l) \rightleftharpoons \mathrm{OH^-}(aq) + \mathrm{HCl}(aq)\] The answer is no. Given that Cl– is a conjugate base to a strong acid (HCl), it is too weak of a base to react with water to produce hydroxide. Also, HCl is a strong acid and essentially does not exist in water. Therefore, we conclude that this reaction cannot occur as HCl would not be generated in an environment where it cannot exist! Given this, hydroxide would not be produced and the pH is not affected by the anion (i.e. a conjugate base to a strong acid).
Conclusion: Ammonium chloride is an acidic salt.
5.8.2 Basic Salts
A basic salt is any salt that raises the pH of a solution. Consider potassium fluoride, KF. The hydrolysis of this salt is given as
\[\mathrm{KF}(s) \overset{\mathrm{H_2O}} \longrightarrow \mathrm{K^+}(aq) + \mathrm{F^-}(aq)\]
Let us consider the reaction of each product of this reaction (i.e. the cation and anion of the salt) with water.
Cation
The potassium ion, K+, is a Group 1A ion and is a conjugate to a strong base, KOH. The following reaction will not occur.
\[\mathrm{K^+}(aq) + \mathrm{2H_2O}(l) \nrightarrow \mathrm{KOH}(s) + \mathrm{H_3O^+}(aq)\]
Anion
Next, consider the anion of the salt. Will the fluoride ion react with water to produce HF?
\[\mathrm{F^-}(aq) + \mathrm{H_2O}(l) \rightleftharpoons \mathrm{OH^-}(aq) + \mathrm{HF}(aq)\]
The answer is yes. Given that F– is a conjugate base to a weak acid (HF), it is a strong enough base to react with water to produce hydroxide. Also, HF is a weak acid and can exist in water. This reaction can occur and given the production of hydroxide, the pH of the solution increases.
Conclusion: Potassium fluoride is a basic salt.
5.8.3 Neutral Salts
Neutral salts are those that do not affect the pH of solution. This means that neither cation or anion of the salt will react with water. Sodium chloride is an excellent example.
\[\mathrm{NaCl}(s) \overset{\mathrm{H_2O}}\longrightarrow \mathrm{Na^+}(aq) + \mathrm{Cl^-}(aq)\]
Cation
The sodium cation is positively charged and stable due to its octet being satisfied. It does not react with water to produce NaH and OH–.
Anion
The chloride anion, as already discussed, is a conjugate base to a strong acid and is therefore too weak to react with water.
Conclusion: Salts containing Group 1A or heavy 2A ions bonded to ions that are conjugates to a strong acid/base are neutral salts.
5.8.4 Acidic Metal Hydrides
Acidic metal hydrides are salts that contain a highly charged metal that, when dissolved, coordinates with water molecules and results in a lower the pH of solution. Highly charged means the metal contains a 3+ (and sometimes 2+) charge. Take for example the soluble aluminum chloride salt, AlCl3.
\[\mathrm{AlCl_3}(s) \overset{\mathrm{H_2O}}\longrightarrow \mathrm{Al^{3+}}(aq) + \mathrm{3Cl^-}(aq)\] The small, highly charged metal coordinates strongly with the partially positive negative oxygen atom in water. There are six coordination sites and, therefore, six coordinating waters in solution. The hydration process is shown below.
\[\mathrm{Al^{3+}}(aq) + \mathrm{6H_2O}(l) \longrightarrow \mathrm{[Al(H_2O)_6]^{3+}}(aq)\]
The product of this reaction is aluminum hexahydrate. This hydrate is a weak acid and can react with water to produce hydronium.
\[\mathrm{[Al(H_2O)_6]^{3+}}(aq) + \mathrm{H_2O}(l) \rightleftharpoons \mathrm{H_3O^+}(aq) + \mathrm{[Al(H_2O)_5OH]^{2+}} \quad K_{\mathrm{a}} = 1.4\times 10^{-5}\]
Conclusion: Salts containing small, high charge metals are acidic salts.
The table below gives the acidities of some metal hydrates.
| Hydrate | Formula | Ka | pKa |
|---|---|---|---|
| Iron hexahydrate | [Fe(H2O)6]3+ |
1.84 × 10-3 |
2.74 |
| Chromium hexahydrate | [Cr(H2O)6]3+ |
1.6 × 10-4 |
3.80 |
| Dimercury dihydrate | [Hg2(H2O)2]2+ |
1 × 10-4 |
4 |
| Aluminum hexahydrate | [Al(H2O)6]3+ |
1.4 × 10-5 |
4.85 |
| Beryllium tetrahydrate | [Be(H2O)4]2+ |
1 × 10 -5 |
5 |
How Do Metal Hydrates Form?
Consider the valence electron configuration for the chromium atom.
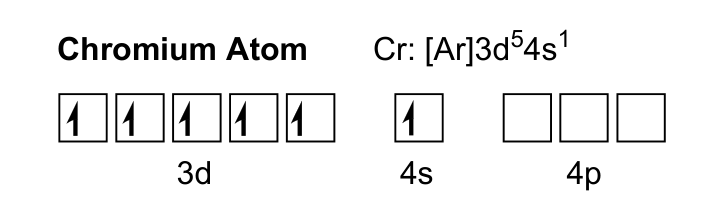
To create Cr3+, the three highest energy electrons are removed, one electron from the 4s orbital and 2 from the 3d orbitals.
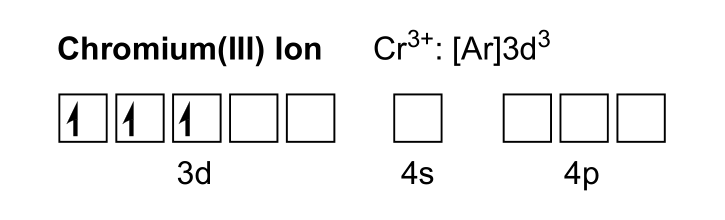
This leaves six unoccupied orbitals in the valence region for chromium. Water has two lone electron pairs on oxygen. A water can donate an electron pair to an empty Cr3+ orbital, forming at dative bond.
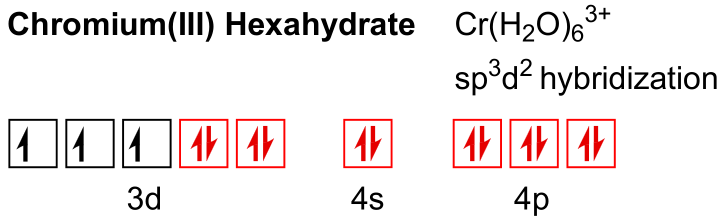
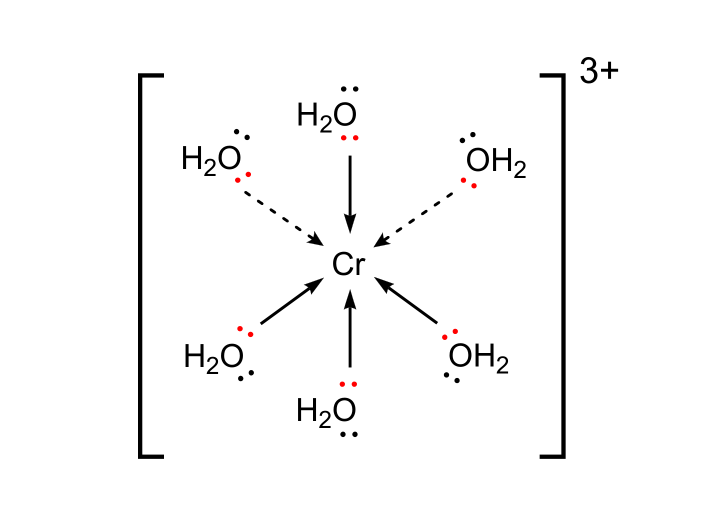
Since six orbitals are unoccupited on Cr3+, six waters can coordinate to the transition metal ion.
5.8.5 Summary
- Acidic salts contain ions that are conjugates to weak bases
- Basic salts contain ions that are conjugates to weak acids
- Group 1A and heavy 2A metal ions do not react with water
- Salts containing high charge metals are acidic salts due to becoming weak acid hydrates
| Ion | Type | Examples |
|---|---|---|
| Acidic cation | Conjugate to weak base | NH4+, CH3NH3+ |
| High charge metal (not 1A or heavy 2A) | Al3+, Fe3+, Cr3+, Sc3+ | |
| Basic anion | Conjugate to weak acid | CN–, NO2–, CH3COO– |
| Neutral cations | Group 1A or heavy Group 2A | Li+, Na+, K+, Ba2+ |
| Neutral anions | Conjugate to strong acids | Cl–, NO3–, ClO4– |