Book Features
Interactive Plots
Plots are made interactive wherever possible and support filtering, zooming, scaling, and more. Rolling over data points provide callout boxes containing the appropriate data. Snapshots can be downloaded as a PNG by hovering over the plot and clicking the camera icon in the toolbar.
Figure 0.1: Concentration vs. time plot for a first-order reaction.
Interactive Objects
Interacting with objects such as geometric shapes can be preferable to static images. Below is an example of a cubic unit cell that can be interacted with using a mouse.
Data Visualization
Wherever appropriate, data is communicated through graphics. Here, the electronegativity of the elements is mapped directly onto the periodic table.
Animations
Data is sometimes better visualized as an animation such as the heating of a substance.
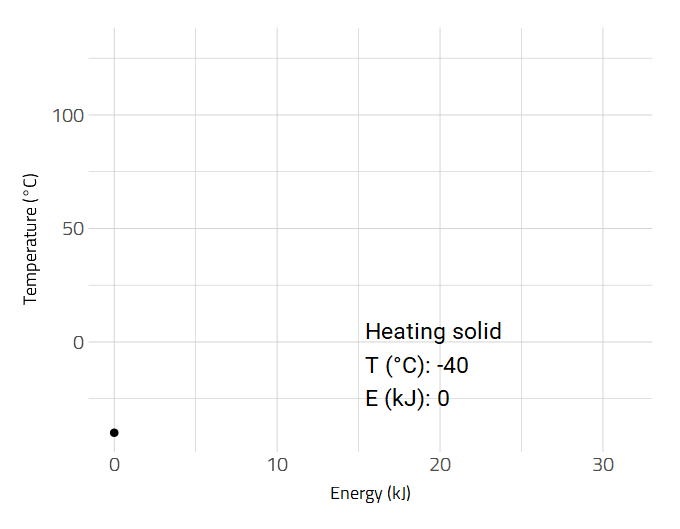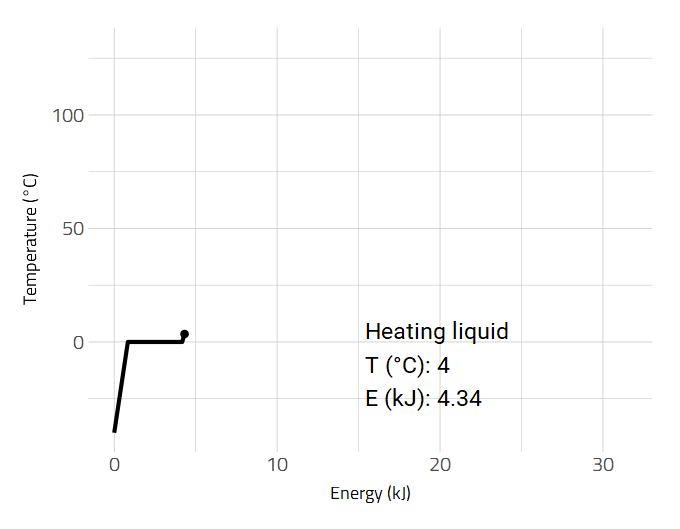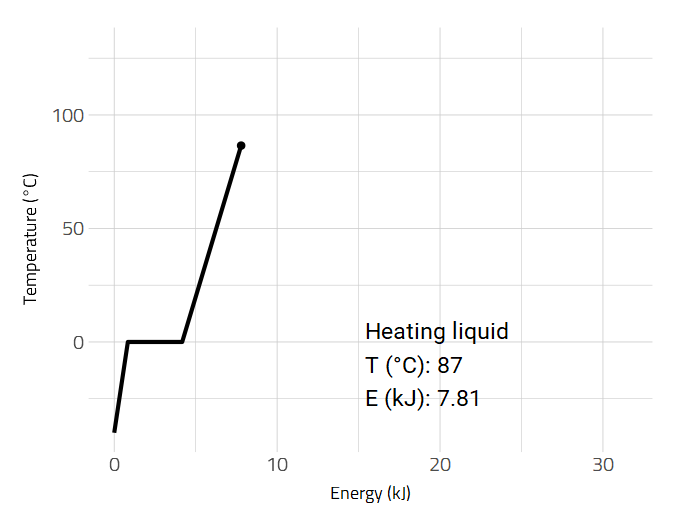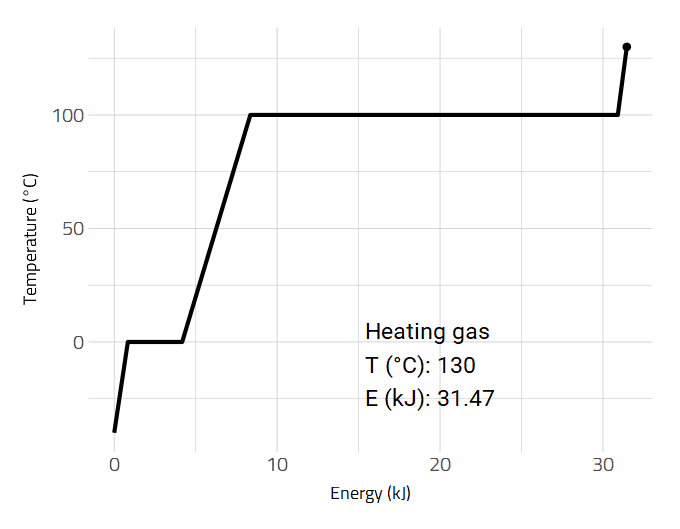
2D and 3D Molecular Representations
Molecules can sometimes be rendered as 2D Lewis-type structures or fully-interactive 3D structures. Benzene is shown below. The 3D structure can be interacted with using a mouse.
LaTeX for Math
LaTeX math is used for beautifully rendered math.
\[\begin{align*} 2\mathrm{NO_2}(g) &\underset{k_{-1}}{\stackrel{k_1}{\rightleftharpoons}} \mathrm{(NO_2)_2}(g) &&\mathrm{(fast)} \\[1.5ex] \mathrm{(NO_2)_2}(g) &\overset{k_2}\longrightarrow \mathrm{2NO}(g) + \mathrm{O_2}(g) &&\mathrm{(slow)} \end{align*}\]
Set the math renderer to “Common HTML” for best results. Right click the LaTeX math above, click “Math Settings”, click “Math Renderer”, then click “Common HTML”.
Practice Problems and Solutions
Practice problems come with step-by-step solutions given in a convenient dropdown box (click “Solution” to view).
Practice
Formic acid has a Ka = 1.9 × 10–4. What is the pKa of the acid?
Solution
\[\begin{align*} \mathrm{p}K_{\mathrm{a}} &= -\log K_{\mathrm{a}} \\[1.5ex] &= -\log (1.9\times 10^{-4}) \\[1.5ex] &= 3.72 \end{align*}\]
End-of-Chapter Practice Problems with Hints
Old exam questions have been provided at the end of each chapter. Some questions have hints which are viewed by clicking on “Hint”.
What is the molecularity for the following elementary step?
\[\mathrm{N_2O}(g) + \mathrm{Cl}(g) \longrightarrow \mathrm{N_2}(g) + \mathrm{ClO}(g)\]
- Unimolecular
- Bimolecular
- Termolecular
And more
- A responsive website
- Integrated search function
- Tabulated data from authoritative sources (where possible)
- Embedded videos
- Math strings for more challenging algebra showing the math expression input for a calculator
To Do:
- Add titles to gganimate vapor pressure curves
- Fix “Error: Expected single logical value” – Bug
- Add energy to acetone curves
- Add ΔH vs T plot – Heat of Vaporization – to Vapor Pressure