2.4 Formation of Solutions
The mixing of substances to form a solution can be endothermic or exothermic. If the final state (i.e. solution) has a lower enthalpy than the initial (non-mixed) state, the mixing process is exothermic.
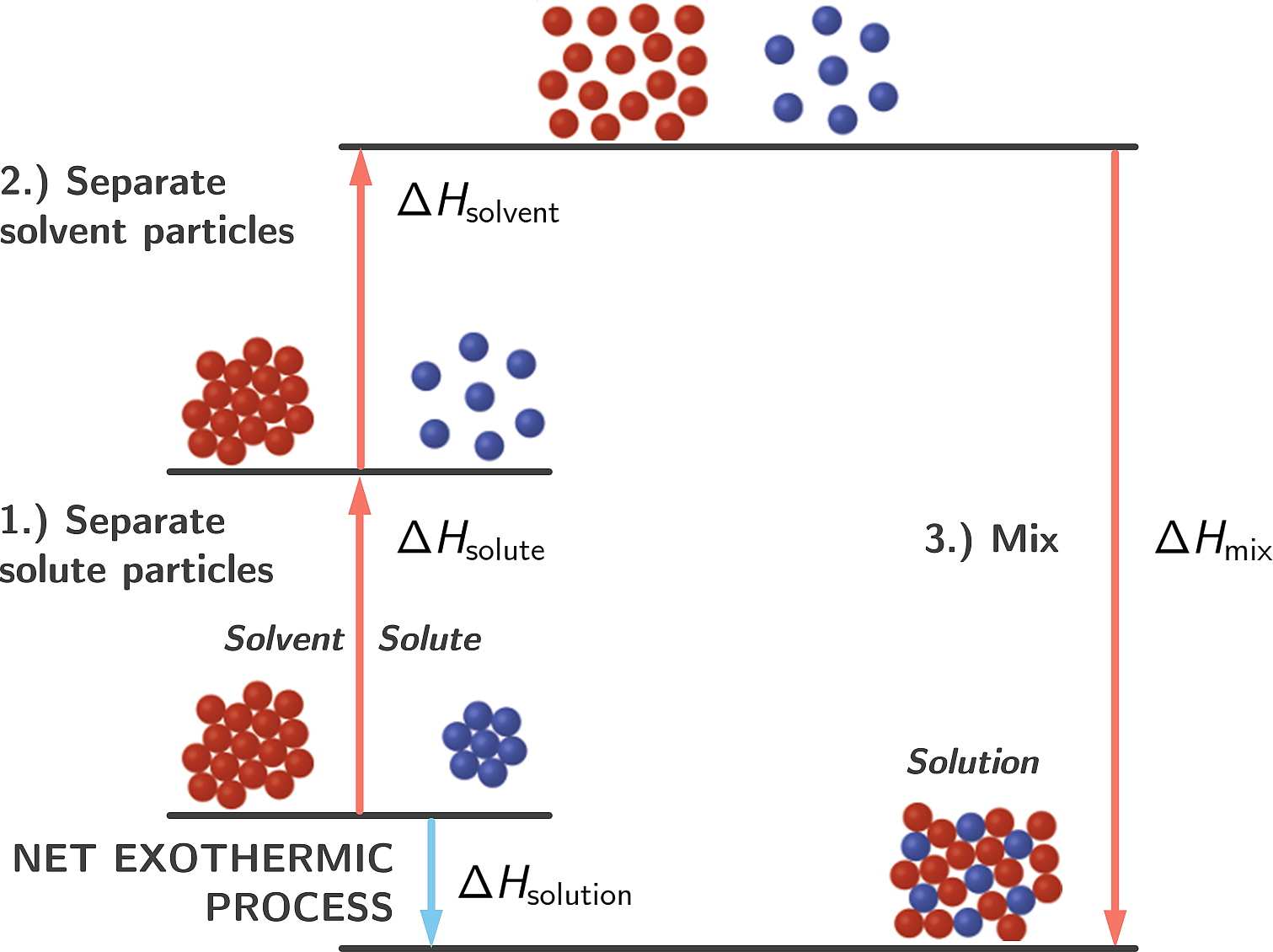
Figure 2.9: Exothermic mixing process
Sulfuric acid + Water
However, if the final state has a higher enthalpy than the initial state, the mixing process is endothermic. This is precisely how cold-packs work. A salt such as ammonium nitrate (NH4NO3) is dissolved in water and heat is absorbed making it feel cold.
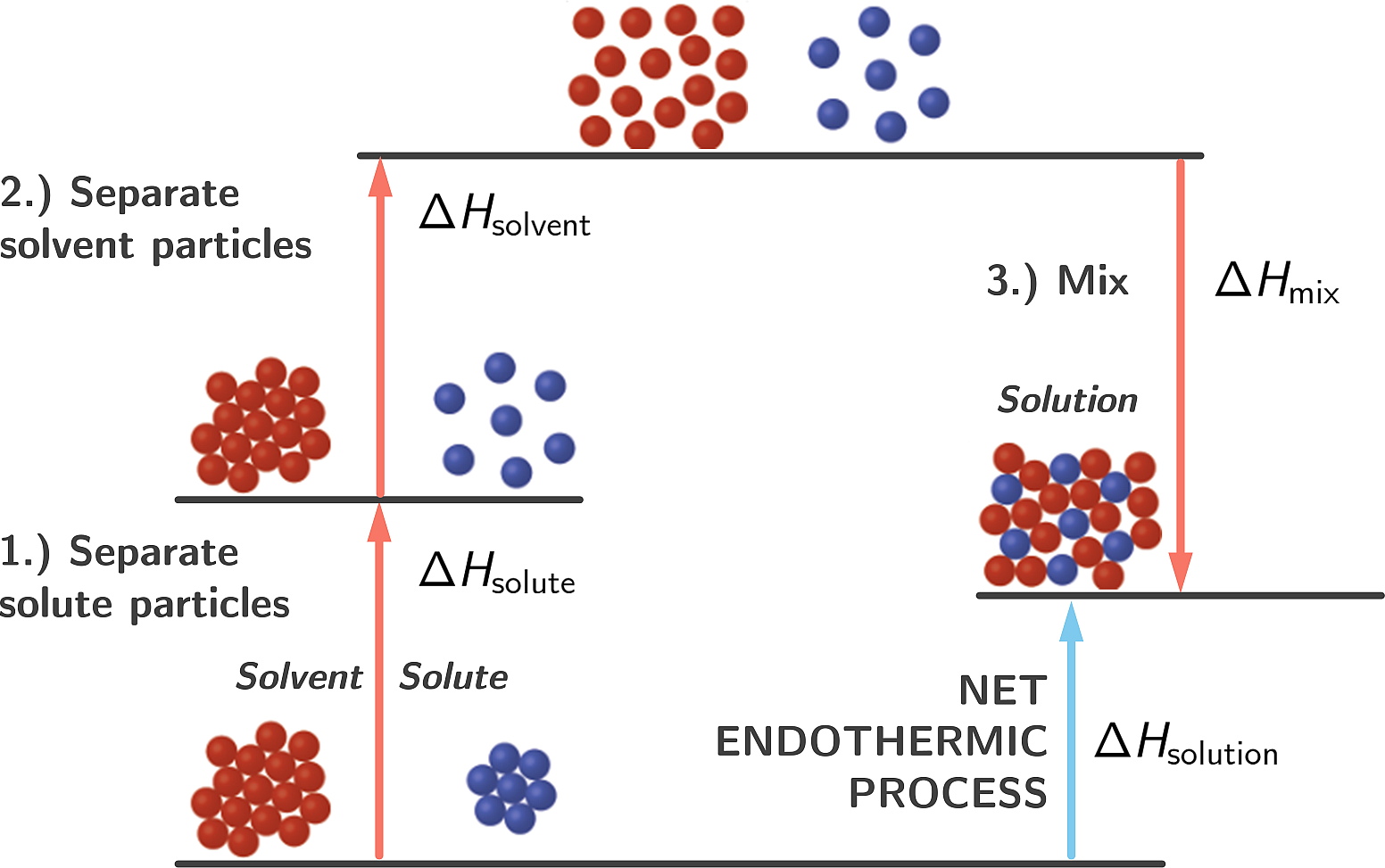
Figure 2.10: Endothermic mixing process
Ammonium chloride + Water