3.7 Collision Theory
Collision theory aims to explain how reactions occur using a collision model. One fundamental principle of collision theory is that two particles must interact (i.e. collide) in order for those two particles to undergo a chemical change. Consider the following reaction:
\[2\mathrm{HI}(g) \longrightarrow \mathrm{H_2}(g) + \mathrm{I_2}(g)\]
Here, two hydrogen iodide molecules must collide to undergo a chemical change that results in the breaking and formation of covalent bonds to produce the products.
A collision of the two molecules on its own does not guarantee that the reactants can become products. The orientation of the two molecules also matter. They must collide in the proper orientation! If the two molecules collide in the orientation shown below, the hydrogen atoms are out of position to potentially form a covalent bond.
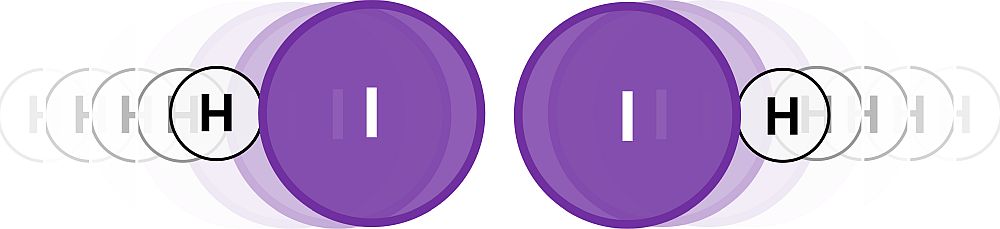
Figure 3.23: An improper collision
However, if the orientation was like this (below), the molecules can react to proceed to product. The transition state is referred to as an “activated complex” and is the geometry the system must have to connect reactants and products together. It has a particular energy just as the reactants and products do. Transition states are higher in energy than the structures they connect.
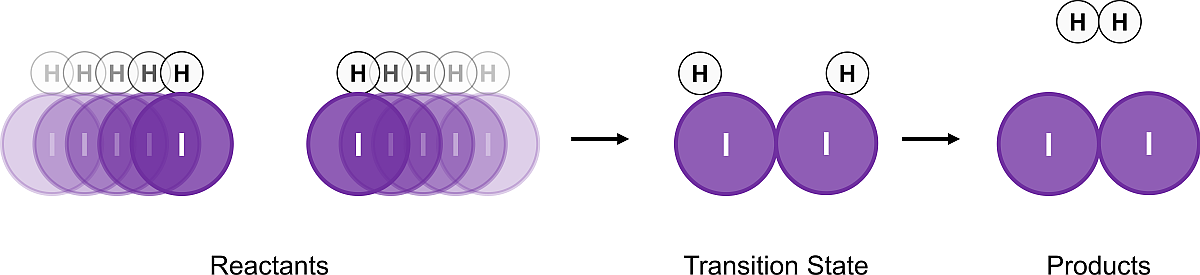
Figure 3.24: An proper collision
In a sample of particles, collisions occur at a certain rate (or frequency) and this rate will increase with increasing temperature. A fraction of the collisions will have the proper orientation and a fraction of those will collide with sufficient energy to move on to products (they must collide with energy equal to or greater than the activation energy of reaction). Collisions that meet all the criteria to facilitate formation of product are referred to as effective collisions. The pre-exponential factor (i.e. Arrhenius factor), A, is correlated with the number of these effective collisions and has the same units as the rate constant.