5.10 Titration Curves
Acid-base titrations is a quantitative analysis technique used to determine the concentration of an acid (or base) in an analyte (the unknown sample) by using a standard solution of base (or acid) to neutralize the unknown sample. The standard solution contains a known concentration of base (or acid). A pH meter is used throughout the titration to track the pH of the solution as base (or acid) is slowly added to the analyte. The resulting plot (pH vs. Vbase added or Vacid added) results in the characteristic titration curve.
5.10.1 Strong Acid + Strong Base
Suppose the analyte contained a strong acid. The pH of the solution is initially very low. The titration curve below indicates a strong acid solution initially (before any strong base is added).
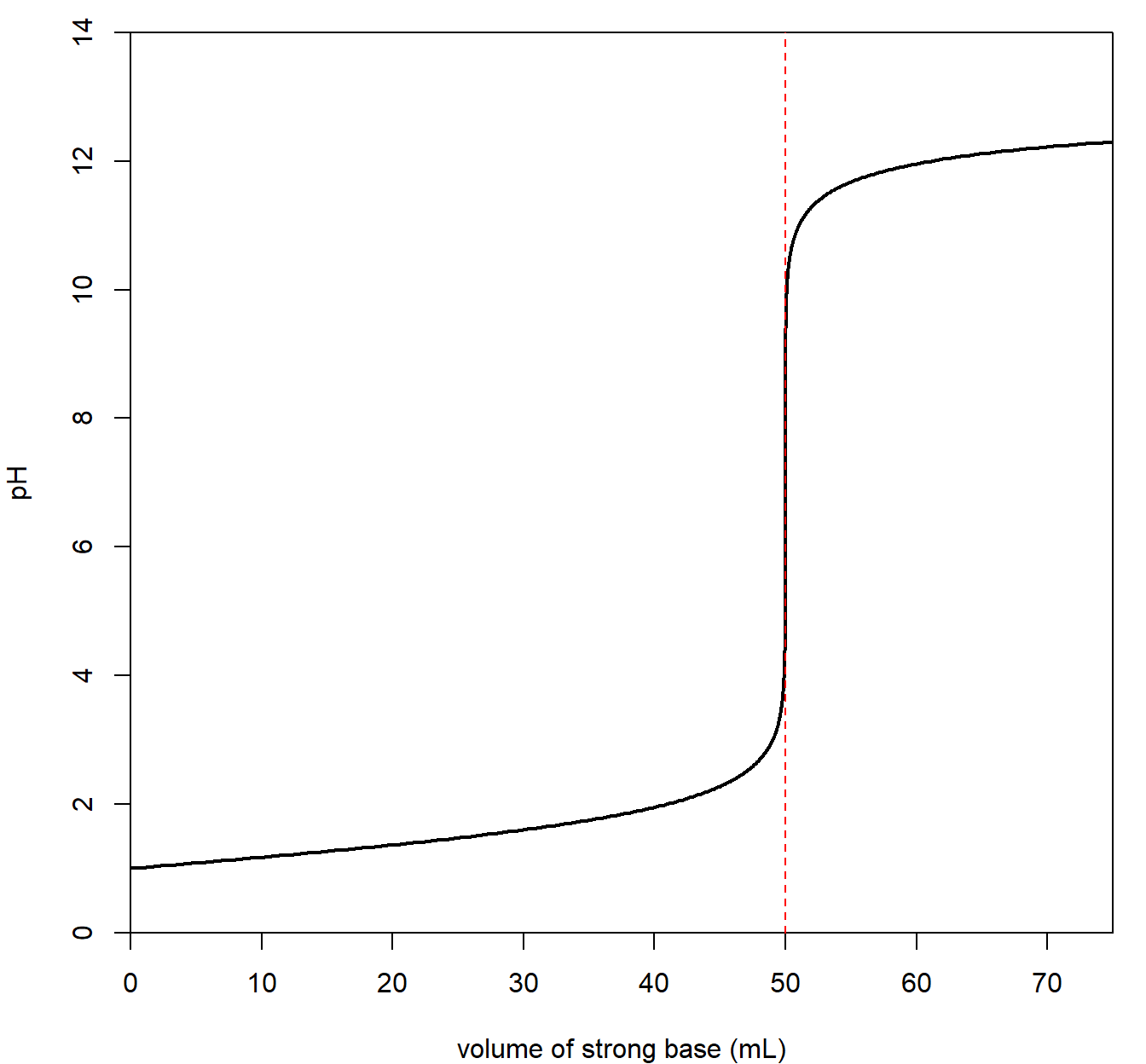
Now, a strong base is slowly added to the analyte. The pH slowly rises as strong base is added. At a particular point, the pH jumps dramatically followed by a slow change in pH as base is added. The point at which the “jump” occurs is called the equivalence point and is the point at which all acid has been neutralized with added base. Therefore, at the equivalence point, the moles of acid equals the moles of base.
A strong acid/strong base titration will result in a pH of 7 at the equivalence point. The final pH of solution is quite high given that a strong base is added to the solution.
5.10.2 Weak Acid + Strong Base
Suppose the analyte contained a weak acid. The pH of the solution is initially somewhat low. The titration curve below indicates a weak acid solution initially (before any strong base is added).
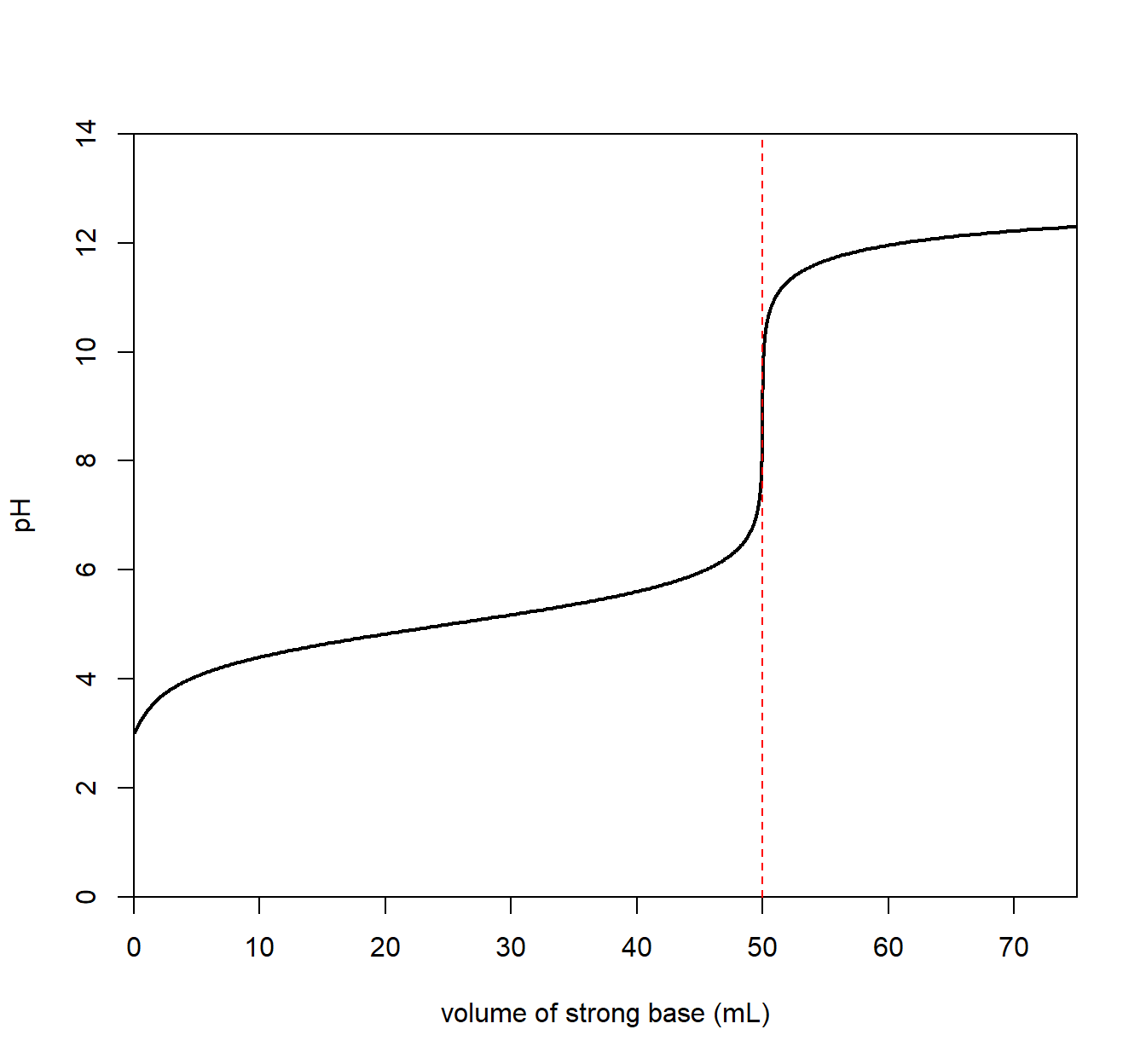
A strong base is slowly added to the analyte. The pH slowly rises as strong base is added. The characteristic equivalence point appears but this time at a pH that is greater than 7. This is a feature of a weak acid/strong base titration.
5.10.3 Strong Base + Strong Acid
Suppose the analyte contained a strong base The pH of the solution is initially very high. The titration curve below indicates a strong acid solution initially (before any strong acid is added).
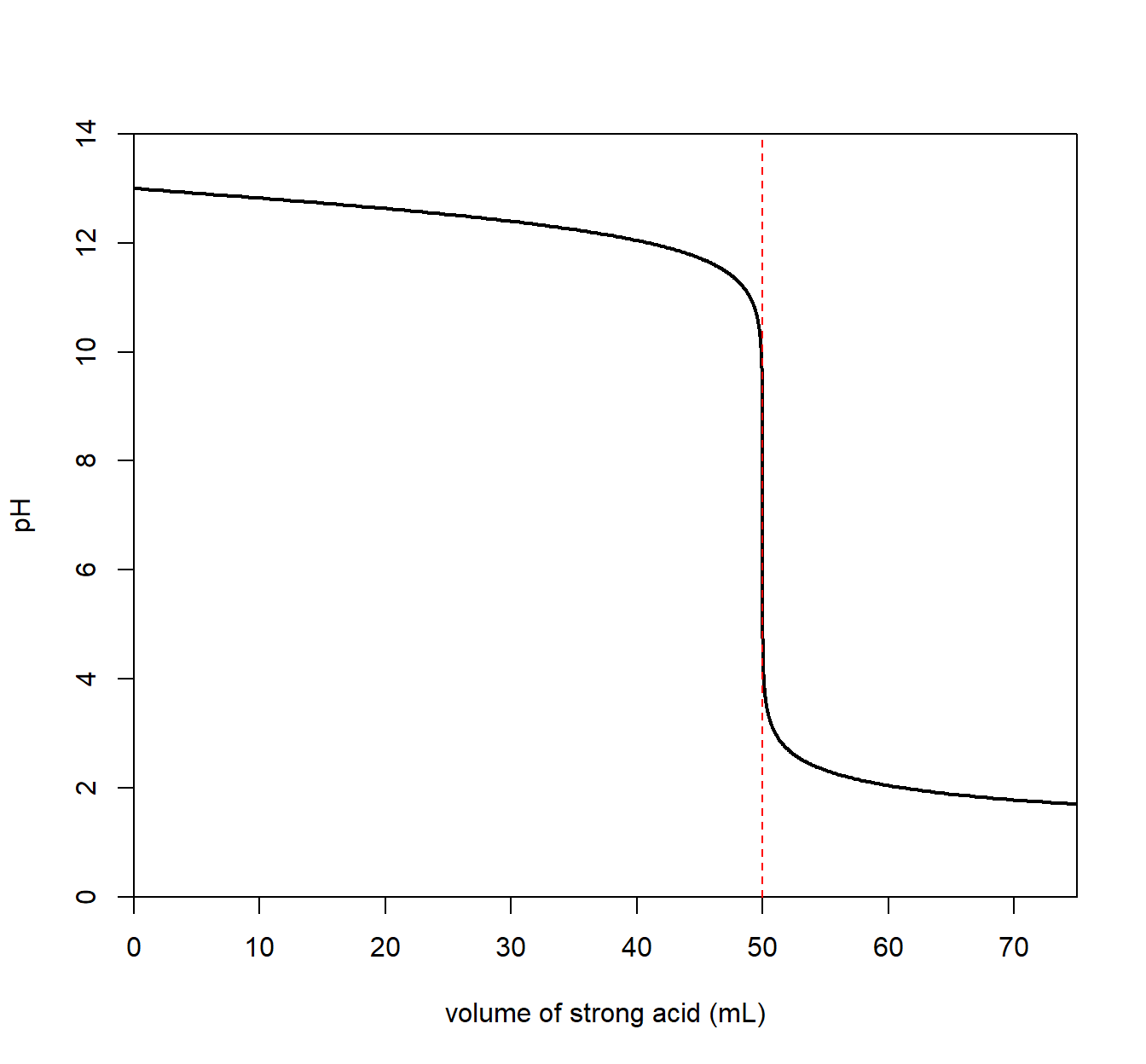
Now, a strong acid is slowly added to the analyte. The pH slowly decreases as strong acid is added. At a particular point, the pH drops dramatically followed by a slow change in pH as base is added. The point at which the “drop” occurs is called the equivalence point and is the point at which all base has been neutralized with added acid.
5.10.4 Weak Base + Strong Acid
Suppose the analyte contained a weak base The pH of the solution is initially somewhat large. The titration curve below indicates a weak base solution initially (before any strong base is added).
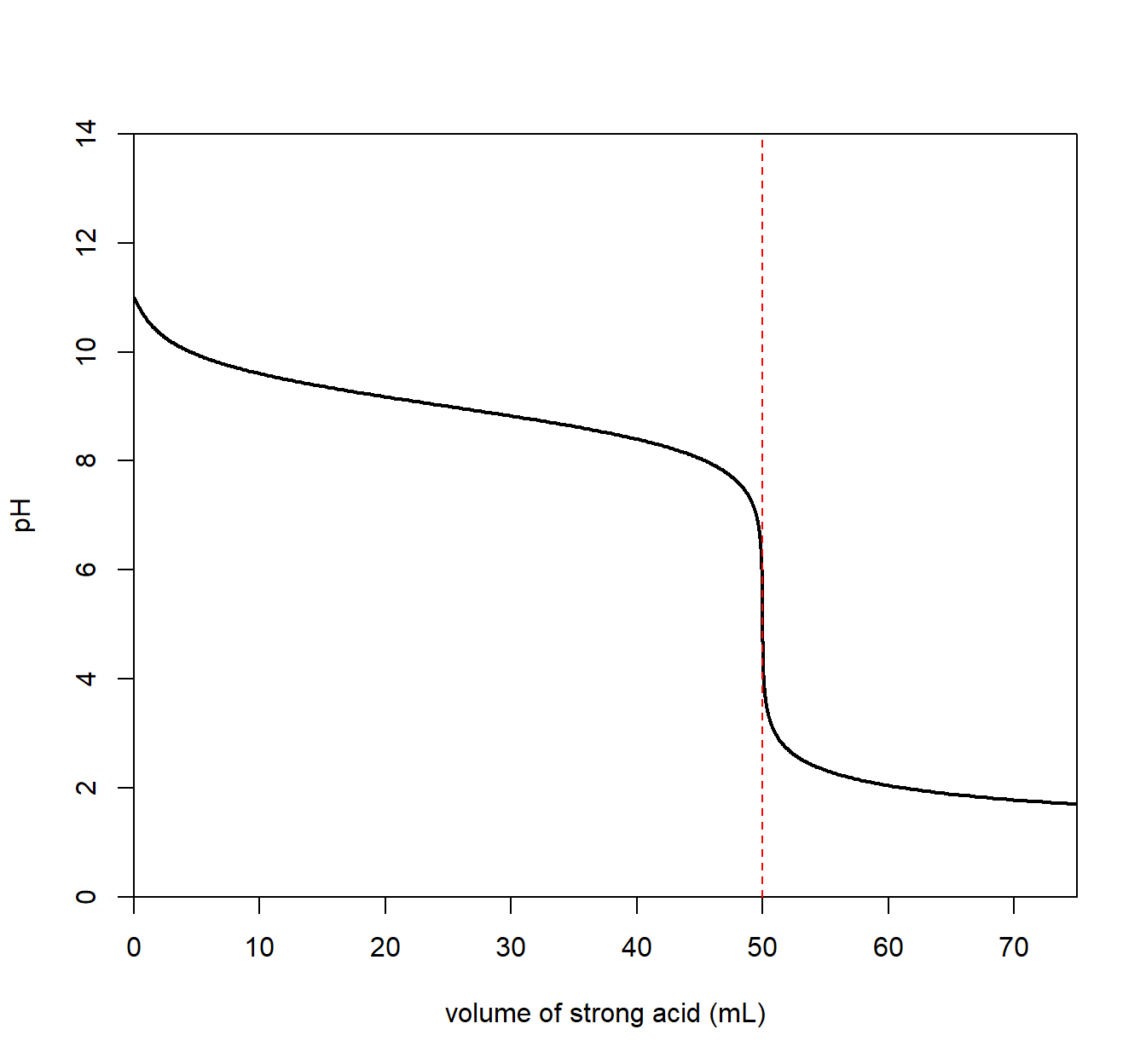
A strong acid is slowly added to the analyte. The pH slowly decreases as strong acid is added. The characteristic equivalence point appears but this time at a pH that is less than 7. This is a feature of a weak base/strong acid titration.
5.10.5 Diprotic Species
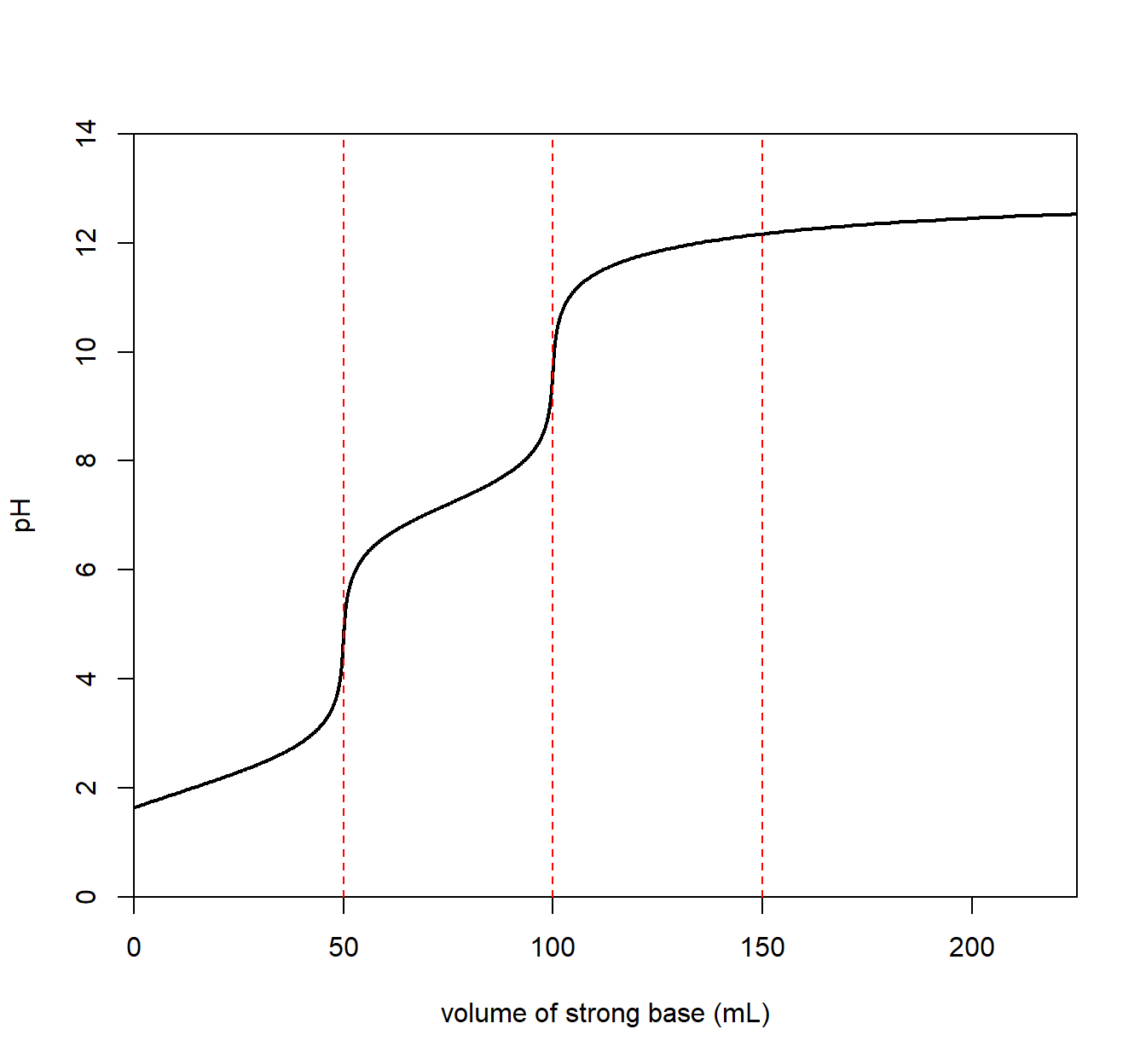
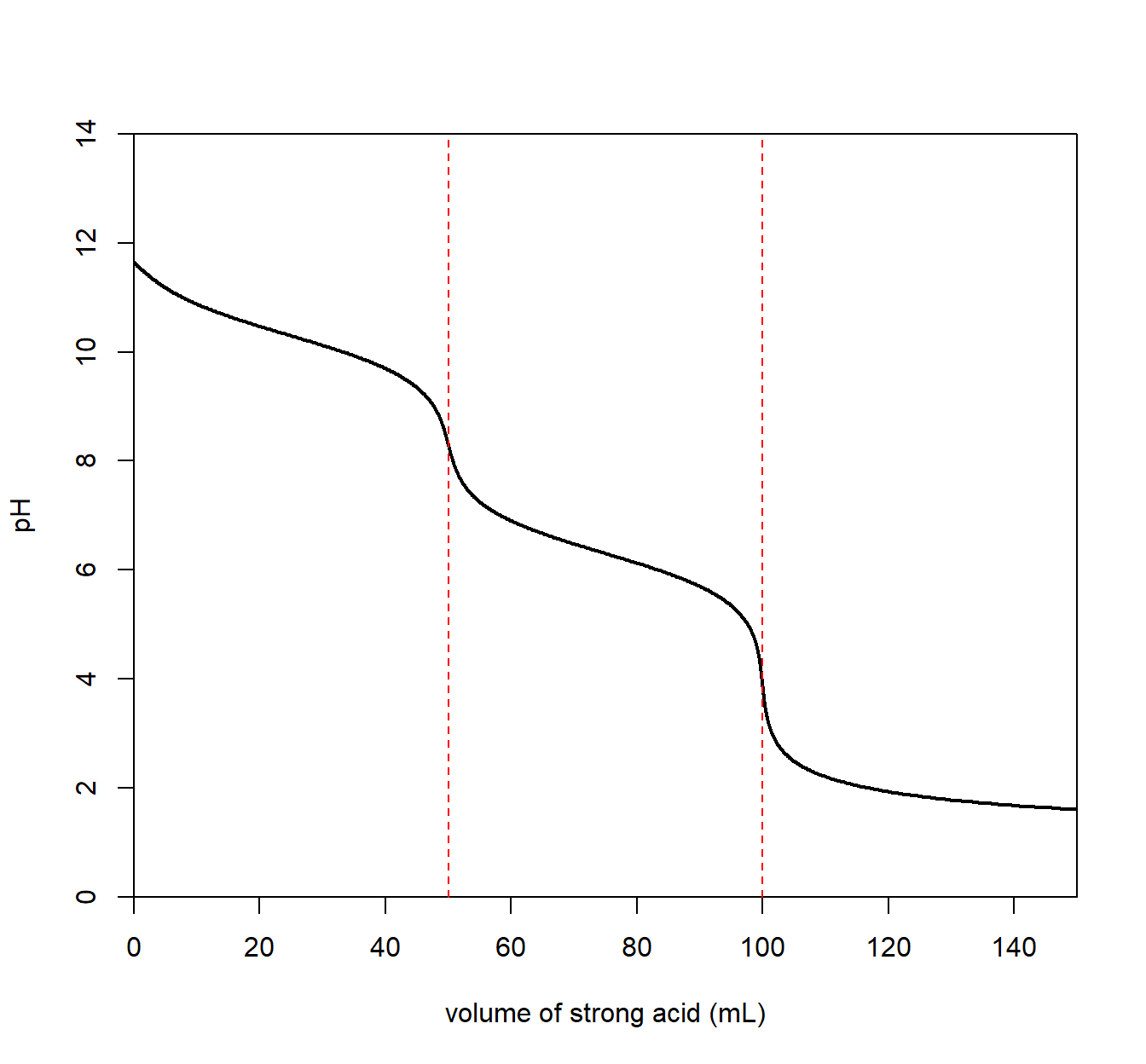
A titration involving a diprotic acid analyte will result in two equivalence points, the first for the first acidic proton and the second for the second acidic proton. The plot below is for carbonic acid (H2CO3) with a pKa1 = 6.3 and pKa2 = 10.33.
We see an equivalence point for a carbonate/bicarbonate (HCO3–) mixture with a pH of about 8.
\[ \mathrm{H_2CO_3}(aq) ~ + ~ \mathrm{H_2O}(l) \longrightarrow \mathrm{H_3O^+}(aq) ~+~ \mathrm{HCO_3^-}(aq) \qquad \mathrm{p}K_{a_1} = 6.3\] The resulting solution is basic since the conjugate base (HCO3–) is a conjugate to a weak acid allowing it to react with water to create OH–.
A second equivalence point is seen with a pH of about 11 for a bicarbonate/carbonic acid where the second proton can pop off.
\[ \mathrm{HCO_3^-}(aq) ~ + ~ \mathrm{H_2O}(l) \longrightarrow \mathrm{H_3O^+}(aq) ~+~ \mathrm{CO_3^{2-}}(aq) \qquad \mathrm{p}K_{a_2} = 10.6\]
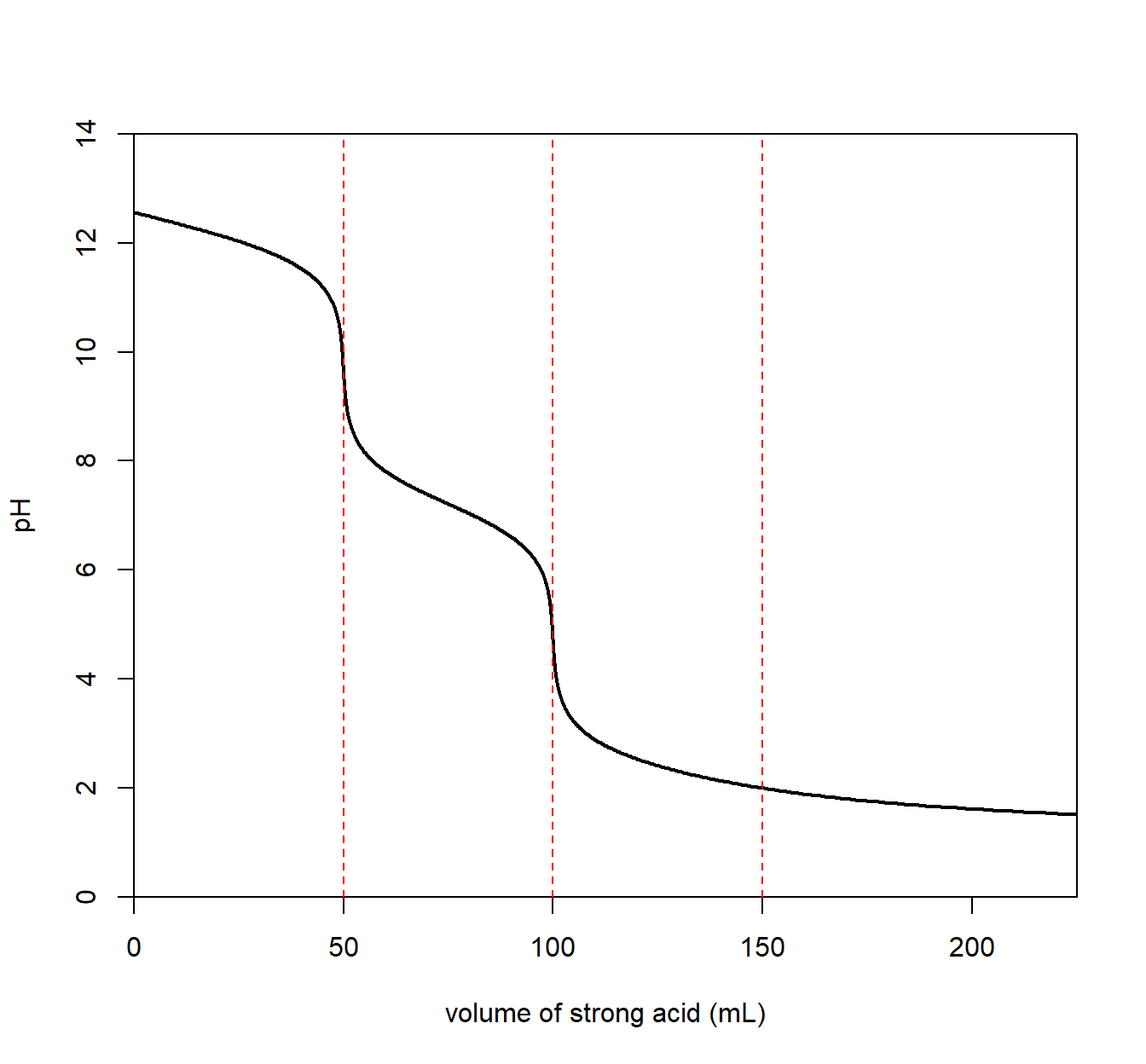
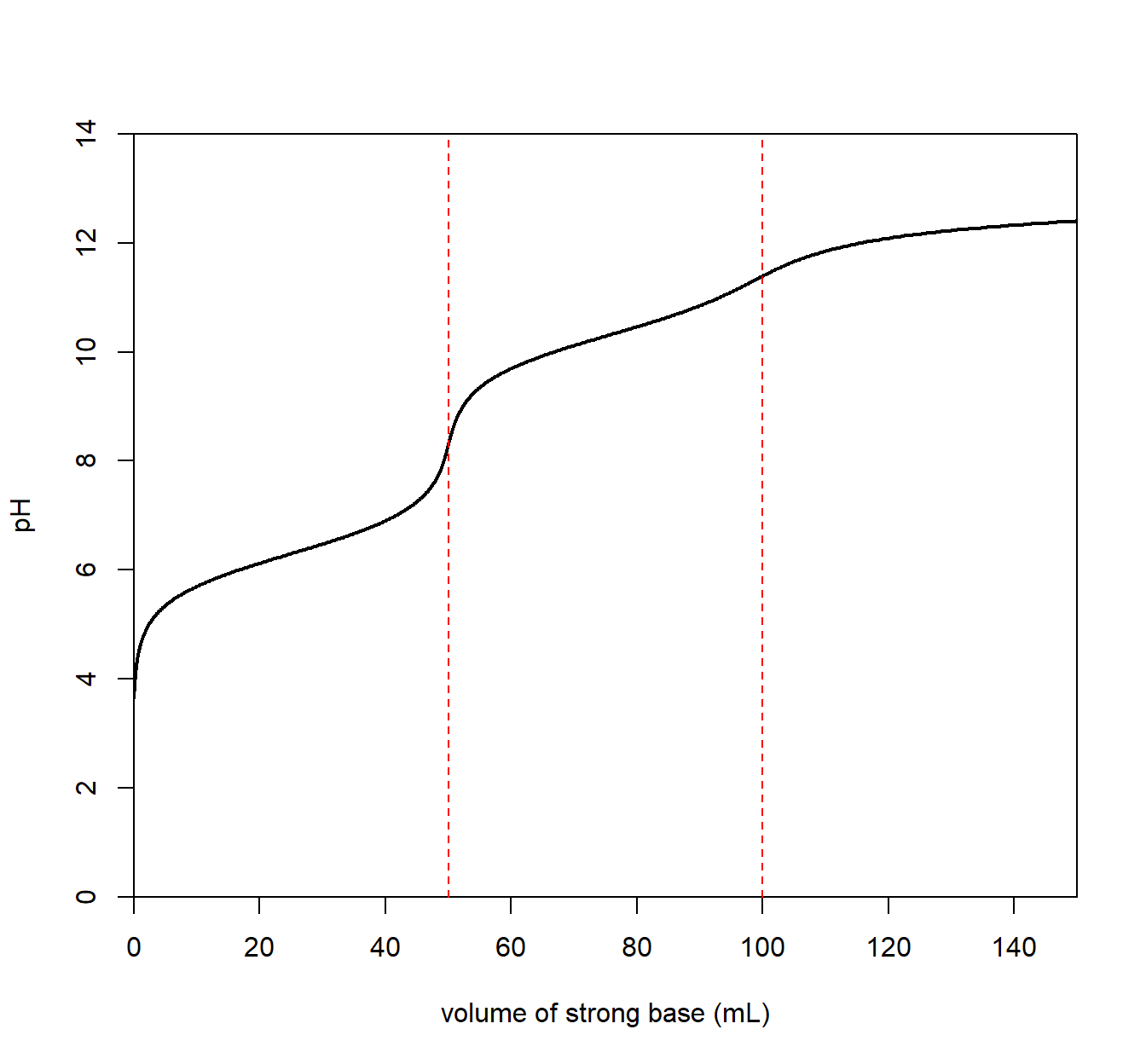
A titration involving a diprotic base analyte will result in two equivalence points. Below is a plot for carbonate dianion (CO32–), a weak, diprotic base.
We see an equivalence point for a carbonate/bicarbonate (HCO3–) mixture with a pH of about 8.
\[\mathrm{CO_3^{2-}}(aq) ~ + ~ \mathrm{H_2O}(l) \longrightarrow \mathrm{OH^-}(aq) ~+~ \mathrm{HCO_3^-}(aq) \qquad \mathrm{p}K_{b_1} = 7.7\] The resulting solution is basic since the conjugate acid (HCO3–) is a conjugate to a weak base (CO32–) allowing it to react with water to create OH–.
A second equivalence point with a pH of about 4 for a bicarbonate/carbonic acid.
\[\mathrm{HCO_3^{-}}(aq) ~ + ~ \mathrm{H_2O}(l) \longrightarrow \mathrm{OH^-}(aq) ~+~ \mathrm{H_2CO_3}(aq) \qquad \mathrm{p}K_{b_2} = 10.4\] The resulting solution is acidic since carbonic acid (H2CO3) is a weak acid and reacts with water to produce H3O+.