1.8 Heating Curves
A heating curve of a substance shows the relationship of temperature, state of matter, and heat (added at a constant rate). Substances undergo phase transitions at their melting and boiling points.
Consider a substance in the solid state below its freezing point.
To convert the substance to a gas above the boiling point, the following must occur:
- Heat solid to its melting point
- Melt the solid from solid to liquid (fusion)
- Heat liquid to its boiling point
- Vaporize liquid to a gas (vaporization)
- Heat gas to the final temperature
Below is an illustration of the heating process for a solid at some initial temperature (Tinitial) to a gas at some final temperature (Tfinal).
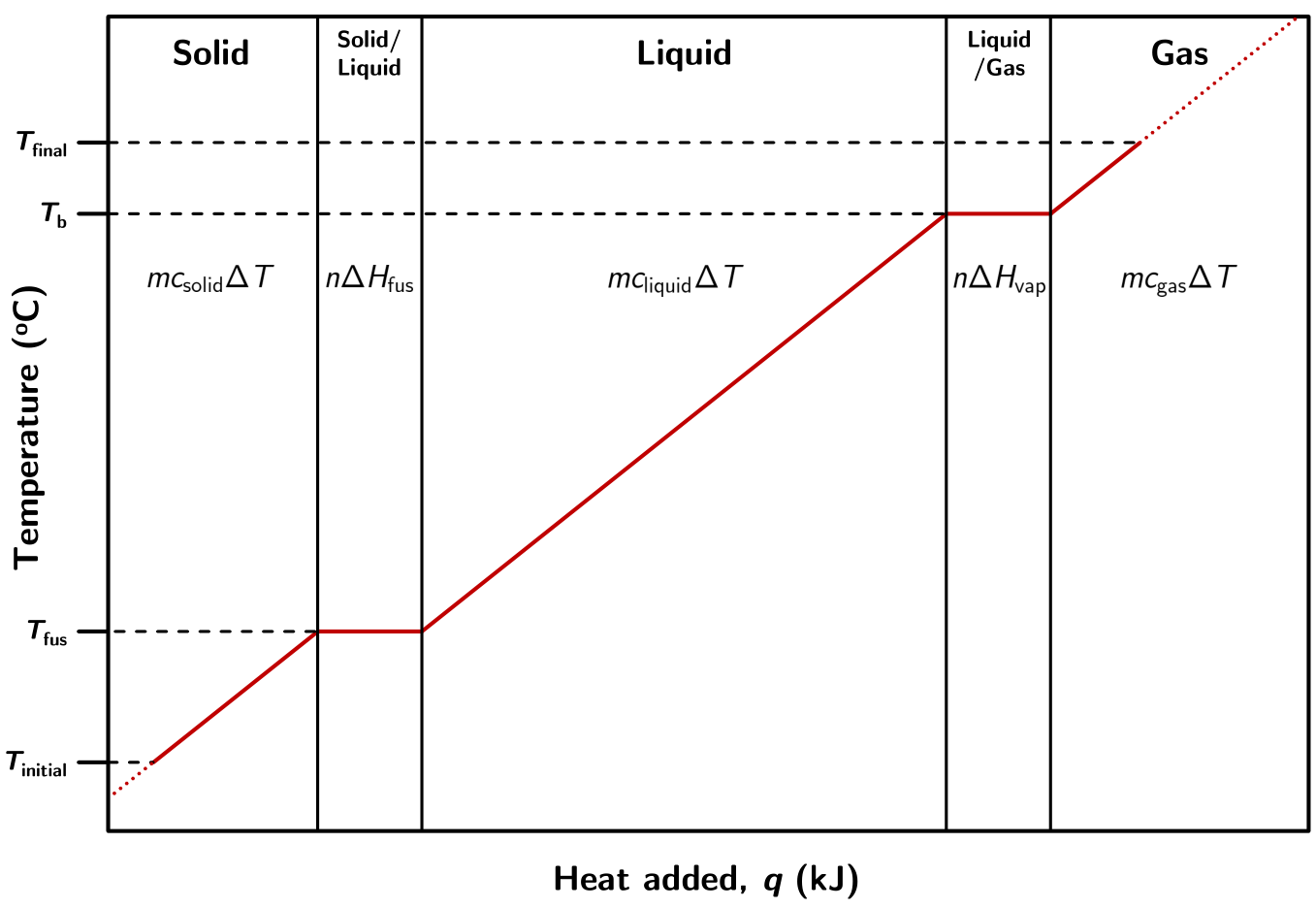
Figure 1.32: A typical heating curve diagram
Consider the heating of 10 g of water from -40 °C to 130 °C (under constant pressure conditions). We recognize that two phase changes will take place, one at the normal melting point of water (Tf = 0 °C) and one at the normal boiling point of water (Tb = 100 °C). We animate this heating process below.
As water is heated, the temperature begins to rise. However, one standout feauture is the constant temperature at the phase transitions (melting and boiling).
The temperature of a substance remains constant during a phase transition.
Let us now compare the heating of varying amounts of water (below). More energy is required to heat larger amounts of water.
Additionally, different substances require different amounts of energy to heat. Below is an animation showing the heating of various amounts of acetone.
We can calculate the energy required to change the temperature of a substance (under constant pressure conditions) by using a couple straightforward equations as well as thermophysical properties of that substance. When a certain amount of substance experiences a temperature change, the amount of heat (q) required to change the temperature of that substance is given as:
\[\begin{equation} q = mc\Delta T \end{equation}\]
where q is the amount of heat, m is the mass of the substance, c is the specific heat of the substance for a given phase (in J g–1 °C–1), and ΔT is the temperature change.
If the specific heat is expressed in J mol–1 K–1, we can rewrite the expression as
\[\begin{equation} q = nc\Delta T \end{equation}\]
where n is the amount of substance in moles. Note that specific heat is state of matter specific. cs is the specific heat of a substance when it is a solid, cl relates to the liquid phase, and cg relates to the gas phase.
The amount of heat required for a certain amount of substance undergoing a phase transition is given as
\[\begin{equation} q = n\Delta H \end{equation}\]
where q is the amount of heat, n is the number of moles of the substance, and ΔH is the enthalpy of that phase transition (generally given in kJ mol–1). ΔHfus is used for the freezing/melting transition while ΔHvap is used for the condensation/vaporization transition.
The table below provides thermophysical values for some substances. Heats of fusion and vaporization (ΔHfus and ΔHvap, respectively, in kJ mol–1) are given at the respective (approximate) normal freezing and boiling points (Tf and Tb in °C) as well as standard temperature (25 °C). Specific heats (c) are given in J g–1 °C–1.
| Substance | Tf | Tb | ΔHfus | ΔHvap | ΔH°vap (25 °C) | cs | cl | cg |
|---|---|---|---|---|---|---|---|---|
Acetone |
-94.9 |
56.08 |
5.77 |
29.1 |
30.99 |
1.653 |
2.409 |
1.283 |
Ammonia |
-77.65 |
-33.33 |
5.66 |
23.33 |
19.86 |
2.061 |
1.703 | |
Benzene |
5.538 |
80.08 |
9.87 |
30.72 |
33.83 |
1.10 |
1.741 |
1.16 |
Butane |
-138.2 |
-0.5 |
4.66 |
22.44 |
21.02 |
2.062 |
1.639 | |
Methanol |
-97.5 |
64.5 |
3.215 |
35.21 |
37.43 |
2.531 |
1.376 | |
Propane |
-187.62 |
-42.11 |
3.5 |
19.04 |
14.79 |
2.025 |
1.669 | |
Water |
0 |
100 |
6.01 |
40.65 |
44.01 |
2.09 |
4.184 |
1.84 |
Small Temperature Dependence
Note that specific heats and heat capacities vary with temperature; however, these changes are generally small across relatively larger changes in temperature (data from CRC4) as shown below.
Figure 1.33: (Isobaric) Heat capacities of some solids vs. temperature
Practice
How much heat (in kJ) is required to heat 10.0 g of water from –40.0 °C to 130.0 °C?
Solution
Water is a solid at –40.0 °C since water melts at 0 °C. Water is a gas at 130.0 °C since water vaporizes at 100 °C.
Heating the solid
\[\begin{align*} q_1 &= mc_{s}\Delta T \\ &= 10~\text{g} \left ( 2.09~\mathrm{J~g^{-1}~^{\circ}C^{-1}} \right ) \left ( 0~^{\circ}\text{C} - -40~^{\circ}\text{C} \right ) \left ( \dfrac{1~\mathrm{kJ}}{10^3~\mathrm{J}} \right ) \\ &= 0.836~\mathrm{kJ} \end{align*}\]
Melting
\[\begin{align*} q_2 &= n\Delta H_{\mathrm{fus}} \\ &= 10~\mathrm{g} \left ( \dfrac{\mathrm{mol}}{18.02~\mathrm{g}} \right ) \left ( \dfrac{6.01~\mathrm{kJ}}{\mathrm{mol}} \right ) \\ &= 3.335~\mathrm{kJ} \end{align*}\]
Heating the liquid
\[\begin{align*} q_3 &= mc_{l}\Delta T \\ &= 10~\text{g} \left ( 4.184~\mathrm{J~g^{-1}~^{\circ}C^{-1}} \right ) \left ( 100~^{\circ}\text{C} - 0~^{\circ}\text{C} \right ) \left ( \dfrac{1~\mathrm{kJ}}{10^3~\mathrm{J}} \right ) \\ &= 4.184 ~\mathrm{kJ} \end{align*}\]
Vaporization
\[\begin{align*} q_4 &= n\Delta H_{\mathrm{vap}} \\ &= 10~\mathrm{g} \left ( \dfrac{\mathrm{mol}}{18.02~\mathrm{g}} \right ) \left ( \dfrac{40.65~\mathrm{kJ}}{\mathrm{mol}} \right ) \\ &= 22.558~\mathrm{kJ} \end{align*}\]
Heating the gas
\[\begin{align*} q_5 &= mc_{g}\Delta T \\ &= 10~\text{g} \left ( 1.84~\mathrm{J~g^{-1}~^{\circ}C^{-1}} \right ) \left ( 130~^{\circ}\text{C} - 100~^{\circ}\text{C} \right ) \left ( \dfrac{1~\mathrm{kJ}}{10^3~\mathrm{J}} \right ) \\ &= 0.552 ~\mathrm{kJ} \end{align*}\]
Total energy
\[\begin{align*} q_{\mathrm{tot}} &= q_1 + q_2 + q_3 + q_4 + q_5 \\ &= 0.836~\mathrm{kJ} + 3.335~\mathrm{kJ} + 4.1874~\mathrm{kJ} + 22.558~\mathrm{kJ} + 0.552~\mathrm{kJ} = 31.47~\mathrm{kJ} \end{align*}\]
Practice
How much heat (in kJ) is required to heat 500.0 g of water from –140.0 °C to 50.0 °C?
Solution
Water is a solid at –140.0 °C since water melts at 0 °C. Water is a liquid at 50.0 °C since water vaporizes at 100 °C.
Heating the solid
\[\begin{align*} q_1 &= mc_{s}\Delta T \\ &= 500~\text{g} \left ( 2.09~\mathrm{J~g^{-1}~^{\circ}C^{-1}} \right ) \left ( 0~^{\circ}\text{C} - -140~^{\circ}\text{C} \right ) \left ( \dfrac{1~\mathrm{kJ}}{10^3~\mathrm{J}} \right ) \\ &= 146.3~\mathrm{kJ} \end{align*}\]
Melting
\[\begin{align*} q_2 &= n\Delta H_{\mathrm{fus}} \\ &= 500~\mathrm{g} \left ( \dfrac{\mathrm{mol}}{18.02~\mathrm{g}} \right ) \left ( \dfrac{6.01~\mathrm{kJ}}{\mathrm{mol}} \right ) \\ &= 166.76~\mathrm{kJ} \end{align*}\]
Heating the liquid
\[\begin{align*} q_3 &= mc_{l}\Delta T \\ &= 500~\text{g} \left ( 4.184~\mathrm{J~g^{-1}~^{\circ}C^{-1}} \right ) \left ( 100~^{\circ}\text{C} - 0~^{\circ}\text{C} \right ) \left ( \dfrac{1~\mathrm{kJ}}{10^3~\mathrm{J}} \right ) \\ &= 209.2 ~\mathrm{kJ} \end{align*}\]
Total energy
\[\begin{align*} q_{\mathrm{tot}} &= q_1 + q_2 + q_3 \\ &= 146.3~\mathrm{kJ} + 166.76~\mathrm{kJ} + 209.2~\mathrm{kJ} = 522.26~\mathrm{kJ} \end{align*}\]
Practice
How much heat (in kJ) is required to heat 500.0 g of acetone from –140.0 °C to 50.0 °C?
Solution
Acetone is a solid at –140.0 °C since acetone melts at –94.9 °C. Acetone is a liquid at 50.0 ° C since acetone vaporizes at 56.08 °C.
Heating the solid
\[\begin{align*} q_1 &= mc_{s}\Delta T \\ &= 500~\text{g} \left ( 1.653~\mathrm{J~g^{-1}~^{\circ}C^{-1}} \right ) \left ( -94.9~^{\circ}\text{C} - -140~^{\circ}\text{C} \right ) \left ( \dfrac{1~\mathrm{kJ}}{10^3~\mathrm{J}} \right ) \\ &= 37.275~\mathrm{kJ} \end{align*}\]
Melting
\[\begin{align*} q_2 &= n\Delta H_{\mathrm{fus}} \\ &= 500~\mathrm{g} \left ( \dfrac{\mathrm{mol}}{58.08~\mathrm{g}} \right ) \left ( \dfrac{5.77~\mathrm{kJ}}{\mathrm{mol}} \right ) \\ &= 49.673~\mathrm{kJ} \end{align*}\]
Heating the liquid
\[\begin{align*} q_3 &= mc_{l}\Delta T \\ &= 500~\text{g} \left ( 2.409~\mathrm{J~g^{-1}~^{\circ}C^{-1}} \right ) \left ( 50~^{\circ}\text{C} - -94.9~^{\circ}\text{C} \right ) \left ( \dfrac{1~\mathrm{kJ}}{10^3~\mathrm{J}} \right ) \\ &= 174.532 ~\mathrm{kJ} \end{align*}\]
Total energy
\[\begin{align*} q_{\mathrm{tot}} &= q_1 + q_2 + q_3 \\ &= 37.275 + 49.673 + 174.532 = 261.48~\mathrm{kJ} \end{align*}\]
Practice
How much heat (in kJ) is required to heat 100 mol of propane from 0 °C to 100.0 °C?
Solution
Propane is a gas at 0.0 °C since propane vaporizes at –42.11 °C.
Heating the gas
\[\begin{align*} q_5 &= mc_{g}\Delta T \\ &= 100~\mathrm{mol} \left ( \dfrac{44.1~\text{g}}{\mathrm{mol}} \right ) \left ( 1.669~\mathrm{J~g^{-1}~^{\circ}C^{-1}} \right ) \left ( 100~^{\circ}\text{C} - 0~^{\circ}\text{C} \right ) \left ( \dfrac{1~\mathrm{kJ}}{10^3~\mathrm{J}} \right ) \\ &= 736.0~\mathrm{kJ} \end{align*}\]
Total energy
\[\begin{align*} q_{\mathrm{tot}} &= q_5 \\ &= 736.0~\mathrm{kJ} \end{align*}\]