5.11 Calculating pH During Titration
When performing a titration, the pH of the solution is monitored via a pH probe, indicator, or some other form. Plotting the pH of solution vs. the amount of titrant added gives the characteristic titration curves (covered above).
We can also calculate the pH of the solution at any point of a titration and reproduce the titration curve plot without ever performing an actual titration!
These types of titration problems involving monoprotic acids can be broken down into four distinct pieces:
- Initial pH
- pH before equivalence point
- pH at the equivalence point
- pH beyond the equivalence point
Strong Acid/Strong Base Curve
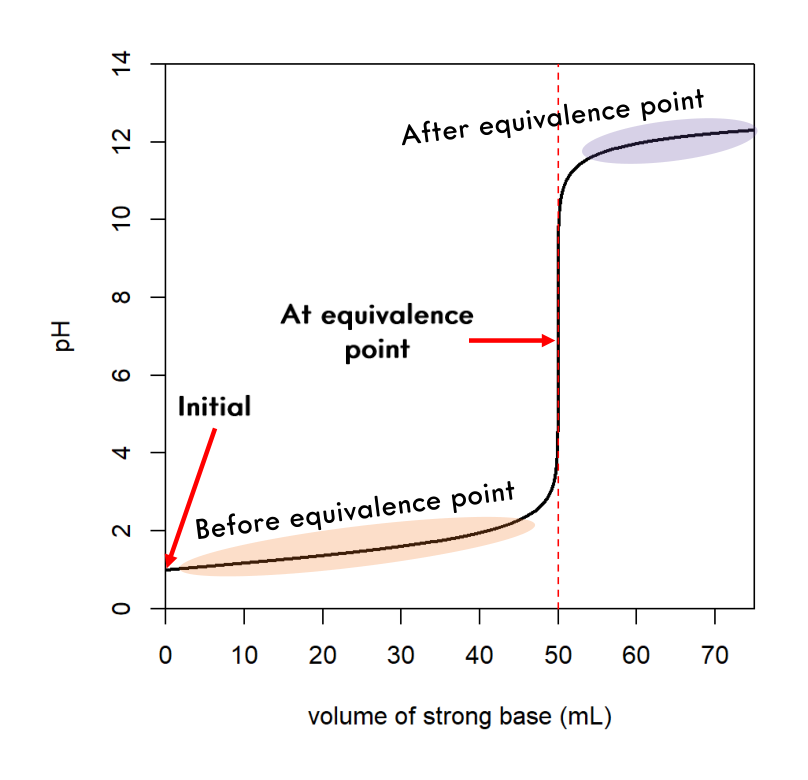
Weak Acid/Strong Base Curve
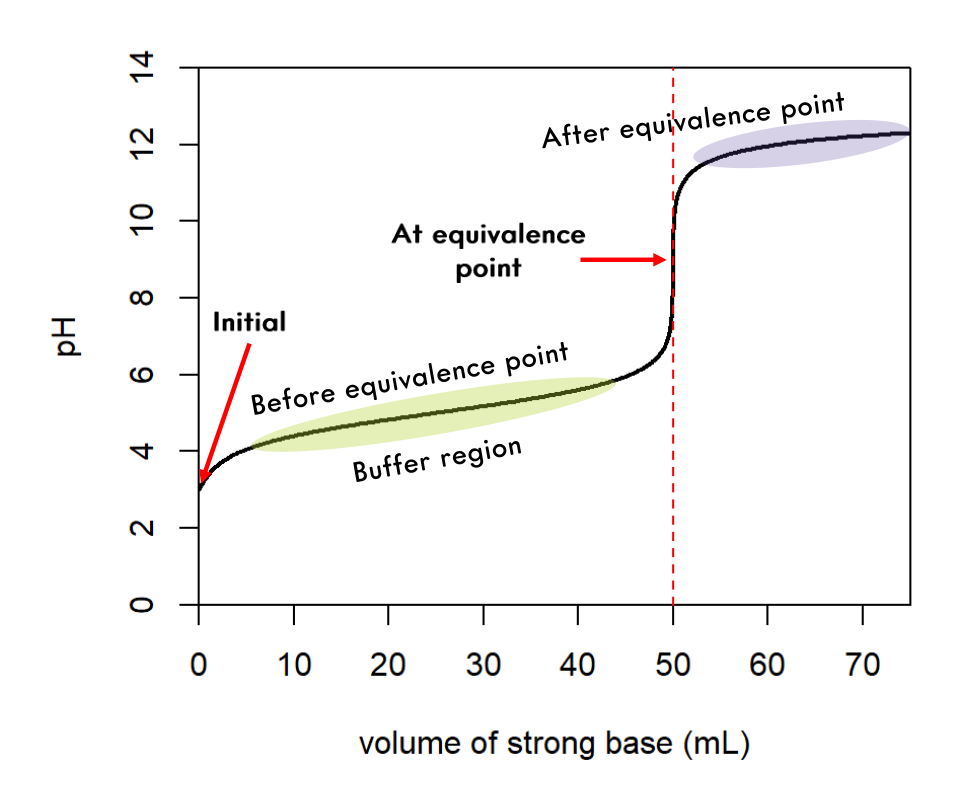
The steps are outlined below.
Step 1: Determine acid/base reaction type
Strong acid/strong base
- \(\mathrm{H^+}(aq) + \mathrm{OH^-}(aq) \longrightarrow \mathrm{H_2O}(l)\)
- \(\mathrm{H_3O^+}(aq) + \mathrm{OH^-}(aq) \longrightarrow \mathrm{2H_2O}(l)\)
Weak acid/strong base
- \(\mathrm{HA}(aq) + \mathrm{OH^-}(aq) \rightleftharpoons \mathrm{H_2O}(l) + \mathrm{A^-}(aq)\)
Strong acid/weak base
- \(\mathrm{H^+}(aq) + \mathrm{B}(aq) \rightleftharpoons \mathrm{HB^+}(aq)\)
- \(\mathrm{H_3O^+}(aq) + \mathrm{B}(aq) \rightleftharpoons \mathrm{HB^+}(aq) + \mathrm{H_2O}(l)\)
Step 2: Determine molar changes (IRF table)
React acid and base (in moles) if applicable
Smaller amount is “wiped out”
Step 3: Determine final pH
If “strong” (H3O+ or OH–) remains
Determine Vfinal
Calculate final [H3O+] or [OH–]
\(\mathrm{pH} = -\log{[\mathrm{H_3O^+}]}\) or \(\mathrm{pOH = -\log[\mathrm{OH^-}]}\)
If “weak” pair present (HA/A– or B/HB+), use Henderson-Hasselbalch
\(\mathrm{pH} = \mathrm{p}K_{\mathrm{a}} + \log\dfrac{[\mathrm{A^-}]}{\mathrm{[HA]}}\)
\(\mathrm{pOH} = \mathrm{p}K_{\mathrm{b}} + \log\dfrac{[\mathrm{HB^+}]}{\mathrm{[B]}}\)
If only one “weak” species present (HA, A–, B, or HB+)
Determine Vfinal
Calculate concentration of “weak” species
Use ICE table to find pH
5.11.1 Strong Acid/Strong Base
Titrate 25.0 mL of 0.100 M HCl with 0.100 M NaOH. (at 25 °C).
Initial pH
What is the initial pH of solution?
Solution
Step 1: Recognize that this is a strong acid/strong base reaction.
Step 2: No molar changes occur since no strong base has been added yet.
Step 3: Determine final pH. Only a “strong” species is present (H3O+).
Given that the initial solution contains a strong acid, recognize that all the strong acid reacts with water to produce hydronium and conjugate base.
\[\mathrm{HCl}(aq) + \mathrm{H_2O}(l) \longrightarrow \mathrm{H_3O^+}(aq) + \mathrm{Cl^-}(aq)\] Therefore, [H3O+] = 0.100 M.
\[\begin{align*} \mathrm{pH} &= -\log [\mathrm{H_3O^+}] \\ &= -\log (0.100) \\ &= 1.00 \end{align*}\]
pH before equivalence point
What is the pH after 15.0 mL of NaOH is added?
Solution
Step 2: Determine molar changes (IRF table)
To determine molar changes, convert concentration (M) to moles
\[\begin{align*} n_{\mathrm{H_3O}^{+}} &= (0.0250~\mathrm{L})\left(0.100~\mathrm{mol~L}^{-1}\right)\\ &= 0.00250~\mathrm{mol} \\[2ex] n_{\mathrm{OH}^{-}} &= (0.0150~\mathrm{L})\left(0.100~\mathrm{mol~L}^{-1}\right)\\ &= 0.00150~\mathrm{mol} \end{align*}\]
React acid and base (in moles).
| Initial moles | 0.00250 | 0.00150 | |||
| Reaction | H3O+ | + | OH– | → | 2H2O |
| Final moles | 0.00100 | 0 |
Step 3: Determine final pH. Only a “strong” species is present (H3O+).
A strong species (H3O+) remains. Find the total volume of solution and convert moles back into molarity (M). Determine pH.
\[\begin{align*} V_{\mathrm{final}} &= 25.0~\mathrm{mL} + 15.0~\mathrm{mL} \\ &= 40.0~\mathrm{mL} \\ &= 0.04~\mathrm{L} \\[2ex] [\mathrm{H_3O^+}] &= \dfrac{0.00100~\mathrm{mol}}{\mathrm{0.040~L}}\\[1.5ex] &= 0.025~M \\[2ex] \mathrm{pH} &= -\log [\mathrm{H_3O^+}] \\ &= -\log (0.025) \\ &= 1.60 \end{align*}\]
pH at the equivalence point
What is the pH at the equivalence point?
Solution
Step 1: Determine acid/base reaction type
- This is a strong acid/strong base titration
- \(\mathrm{H_3O^+}(aq) + \mathrm{OH^-}(aq) \longrightarrow \mathrm{2H_2O}(l)\)
Step 2: Determine molar changes (IRF table)
To determine molar changes, convert concentration (M) to moles
\[\begin{align*} n_{\mathrm{H_3O}^{+}} &= (0.0250~\mathrm{L})\left(0.100~\mathrm{mol~L}^{-1}\right)\\ &= 0.00250~\mathrm{mol} \end{align*}\]
Note that a volume of added base was not given. However, we implement the definition of the equivalence point (nacid = nbase) and recognize that there must be an equal number of moles of base as there is acid! Therefore,
\[\begin{align*} n_{\mathrm{OH^-}} &= 0.00250~\mathrm{mol} \\[2ex] \end{align*}\]
React acid and base (in moles).
| Initial moles | 0.00250 | 0.00250 | |||
| Reaction | H3O+ | + | OH– | → | 2H2O |
| Final moles | 0 | 0 |
Step 3: Determine final pH
Since no strong species (H3O+ or OH–) remains, the solution is neutral. All acid and base was converted to water. The only hydronium and hydroxide ions in solution come from pure water via the autoionization of water (at 25 °C)
\[2\mathrm{H_2O}(l) \rightleftharpoons \mathrm{H_3O^+}(aq) + \mathrm{OH^-}(aq)\quad K_{\mathrm{w}} = 1.00\times 10^{-14}\]
\[\begin{align*} [\mathrm{H_3O^+}] &= [\mathrm{OH^-}] = 1\times 10^{-7}\\[2ex] \mathrm{pH} &= [\mathrm{H_3O^+}] \\ &= -\log (1\times 10^{-7}) \\ &= 7 \end{align*}\]
pH beyond the equivalence point
What is the pH after 30.0 mL of NaOH is added?
Solution
Step 1: Determine acid/base reaction type
- This is a strong acid/strong base titration
- \(\mathrm{H_3O^+}(aq) + \mathrm{OH^-}(aq) \longrightarrow \mathrm{2H_2O}(l)\)
Step 2: Determine molar changes (IRF table)
To determine molar changes, convert concentration (M) to moles
\[\begin{align*} n_{\mathrm{H_3O}^{+}} &= (0.0250~\mathrm{L})\left(0.100~\mathrm{mol~L}^{-1}\right)\\ &= 0.00250~\mathrm{mol} \\[2ex] n_{\mathrm{OH}^{-}} &= (0.0300~\mathrm{L})\left(0.100~\mathrm{mol~L}^{-1}\right)\\ &= 0.00300~\mathrm{mol} \end{align*}\]
React acid and base (in moles).
| Initial moles | 0.00250 | 0.00300 | |||
| Reaction | H3O+ | + | OH– | → | 2H2O |
| Final moles | 0 | 0.0005 |
Step 3: Determine final pH
A strong species (OH–) remains. Find the total volume of solution and convert moles back into molarity (M). Get pH.
\[\begin{align*} V_{\mathrm{final}} &= 25.0~\mathrm{mL} + 30.0~\mathrm{mL} \\ &= 55.0~\mathrm{mL}\\ &= 0.055~\mathrm{L} \\[2ex] [\mathrm{OH^-}] &= \dfrac{0.0005~\mathrm{mol}}{\mathrm{0.055~L}} \\[1.5ex] &= 0.0091~M \\[2ex] \mathrm{pOH} &= -\log [\mathrm{OH^-}] \\ &= -\log (0.0091) = 2.04 \\[2ex] \mathrm{pH} &= \mathrm{p}K_{\mathrm{w}} - \mathrm{pOH}\\[1ex] &= 14 - 2.04 \\[1ex] &= 11.96 \end{align*}\]
5.11.2 Weak Acid/Strong Base
Titrate 25.0 mL of 0.100 M CH3COOH with 0.100 M NaOH (at 25 °C).
Ka(CH3OOH) = 1.80 × 10–5
Initial pH
What is the initial pH of solution?
Solution
Step 1: Determine acid/base reaction type
- This is a weak acid/strong base titration
Step 2: No molar changes occur since no strong base has been added yet.
Step 3: Determine final pH
This is a typical equilibrium problem via an ICE table.
| CH3OOH | + | H2O | ⇌ | H3O+ | + | CH3OO– | |
|---|---|---|---|---|---|---|---|
| I | 0.100 | ≈0 | 0 | ||||
| C | –x | +x | +x | ||||
| E | 0.100 - x | x | x |
Solve the equilibrium expression.
\[\begin{align*} \dfrac{[\mathrm{H_3O^+}][\mathrm{A^-}]}{\mathrm{[HA]}} &= K_{\mathrm{a}} \\[1.5ex] \dfrac{x^2}{0.100-x} &= 1.80\times 10^{-5}\\[1.5ex] \dfrac{x^2}{0.100} &= 1.80\times 10^{-5}\\[1.5ex] x &= 1.34\times 10^{-3}\\[2ex] \dfrac{1.34\times 10^{-3}}{0.100} \times 100\% &= 1.34\% \end{align*}\]
Get pH. \[\begin{align*} \mathrm{pH} &= -\log[\mathrm{H_3O^+}] \\ &= -\log (1.3\times 10^{-3}) \\ &= 2.87 \end{align*}\]
pH before equivalence point
What is the pH after 15.0 mL of NaOH is added?
Solution
Step 1: Determine acid/base reaction type
- This is a weak acid/strong base titration
- \(\mathrm{HA}(aq) + \mathrm{OH^-}(aq) \rightleftharpoons \mathrm{H_2O}(l) + \mathrm{A^-}(aq)\)
Step 2: Determine molar changes (IRF table)
To determine molar changes, convert concentration (M) to moles
\[\begin{align*} n_{\mathrm{HA}} &= (0.0250~\mathrm{L})\left(0.100~\mathrm{mol~L}^{-1}\right)\\ &= 0.00250~\mathrm{mol} \\[2ex] n_{\mathrm{OH}^{-}} &= (0.0150~\mathrm{L})\left(0.100~\mathrm{mol~L}^{-1}\right)\\ &= 0.00150~\mathrm{mol} \end{align*}\]
React acid and base (in moles).
| Initial moles | 0.00250 | 0.00150 | 0 | ||||
| Reaction | HA | + | OH– | → | H2O | + | A– |
| Final moles | 0.00100 | 0 | 0.00150 |
Step 3: Determine final pH
If “weak” pair present (HA/A– or B/HB+), determine of this is a buffer. If it is, use Henderson-Hasselbalch to get the pH.
\[\begin{align*} \dfrac{[\mathrm{A^-}]}{[\mathrm{HA}]} = \dfrac{0.00150}{0.00100} = 1.5 \end{align*}\]
or
\[\begin{align*} \dfrac{[\mathrm{HA}]}{[\mathrm{A^-}]} = \dfrac{0.00100}{0.00150} = 0.667 \end{align*}\]
Since the ratio of acid to conjugate base is between 0.1 and 10, we have a buffer and can use Henderson-Hasselbalch!
\[\begin{align*} \mathrm{pH} &= \mathrm{p}K_{\mathrm{a}} + \log\dfrac{[\mathrm{A^-}]}{\mathrm{[HA]}} \\[1ex] &= -\log(1.80\times 10^{-5}) + \log\dfrac{0.00150}{0.00100} \\[1ex] &= 4.92 \end{align*}\]
Why did you use moles??
I used mole quantities here in the Henderson-Hasselbalch equation because converting to concentration would net the same answer. Consider the two examples:
Example 1: Mole quantities
\[\begin{align*} \mathrm{pH} &= \mathrm{p}K_{\mathrm{a}} + \log\dfrac{[\mathrm{A^-}]}{\mathrm{[HA]}} \\[1.5ex] &= -\log(1.80\times 10^{-5}) + \log\dfrac{0.00150}{0.0010} \\[1.5ex] &= 4.92 \end{align*}\]
Example 2: Molar concentration quantities
\[V_{\mathrm{tot}} = 25.0~\mathrm{mL} + 15.0~\mathrm{mL} = 40.0~\mathrm{mL}\]
\[\begin{align*} [\mathrm{H_3O^+}] &= \dfrac{0.00100~\mathrm{mol~H_3O^+}}{0.040~\mathrm{L}}\\[1.5ex] &= 0.0250~M\\[2ex] [\mathrm{OH^-}] &= \dfrac{0.00150~\mathrm{mol~A^-}}{0.040~\mathrm{L}}\\[1.5ex] &= 0.0375~M \end{align*}\]
\[\begin{align*} \mathrm{pH} &= \mathrm{p}K_{\mathrm{a}} + \log\dfrac{[\mathrm{A^-}]}{\mathrm{[HA]}} \\ &= -\log(1.80\times 10^{-5}) + \log\dfrac{0.0375}{0.0250} \\ &= 4.92 \end{align*}\]
pH at equivalence point
What is the pH at the equivalence point?
Solution
Step 1: Determine acid/base reaction type
- This is a weak acid/strong base titration
- \(\mathrm{HA}(aq) + \mathrm{OH^-}(aq) \rightleftharpoons \mathrm{H_2O}(l) + \mathrm{A^-}(aq)\)
Step 2: Determine molar changes (IRF table)
To determine molar changes, convert concentration (M) to moles
\[\begin{align*} n_{\mathrm{HA}} &= (0.0250~\mathrm{L})\left(0.100~\mathrm{mol~L}^{-1}\right)\\ &= 0.00250~\mathrm{mol} \\[2ex] \end{align*}\]
Note that a volume of added base was not given. However, we implement the definition of the equivalence point (nacid = nbase) and recognize that there must be an equal number of moles of base as there is acid! Therefore,
\[\begin{align*} n_{\mathrm{OH^-}} &= 0.00250~\mathrm{mol} \\[2ex] \end{align*}\]
React acid and base (in moles).
| Initial moles | 0.00250 | 0.00250 | 0 | ||||
| Reaction | HA | + | OH– | → | H2O | + | A– |
| Final moles | 0 | 0 | 0.00250 |
Step 3: Determine final pH
Only one “weak” species (A–) is present. Convert moles back into molarity (M).
First, determine the volume of base added.
\[\begin{align*} V_{\mathrm{NaOH}} &= \dfrac{0.00250~\mathrm{mol}}{0.100~\mathrm{mol~L^{-1}}} \\[1.5ex] &= 0.025~\mathrm{L} \\[1.5ex] &= 25.0~\mathrm{mL} \end{align*}\]
Next, find the total volume of solution and determine the concentration of A–.
\[\begin{align*} V_{\mathrm{final}} &= 25.0~\mathrm{mL} + 25.0~\mathrm{mL} \\ &= 50.0~\mathrm{mL} \\ &= 0.0500~\mathrm{L} \\[2ex] [\mathrm{A^-}] &= \dfrac{0.00250~\mathrm{mol}}{0.0500~\mathrm{L}}\\[1.5ex] &= 0.0500~M \end{align*}\]
Set up an ICE table for A–, a conjugate base, reacting with water.
| A– | + | H2O | ⇌ | OH– | + | HA | |
|---|---|---|---|---|---|---|---|
| I | 0.0500 | ≈0 | 0 | ||||
| C | –x | +x | +x | ||||
| E | 0.0500 - x | x | x |
NOTE: Because we are analyzing a base-ionization reaction (since we have a base reacting with water), you must convert the acid-dissociation constant, Ka, to the base-ionization constant, Kb, of the corresponding conjugate base!
\[\begin{align*} K_{\mathrm{b}} &= \dfrac{K_{\mathrm{w}}}{K_{\mathrm{a}}} \\[1.5ex] &= \dfrac{1.00\times 10^{-14}}{1.80\times 10^{-5}} \\[1.5ex] &= 5.6\times 10^{-10} \end{align*}\]
Set up the equilibrium expression and solve.
\[\begin{align*} \dfrac{[\mathrm{OH^-}][\mathrm{HA}]}{[\mathrm{A^-}]} &= K_{\mathrm{b}}\\[1.5ex] \dfrac{x^2}{0.0500-x} &= 5.6\times 10^{-10}\\[1.5ex] \dfrac{x^2}{0.0500} &= 5.6\times 10^{-10} \\[1.5ex] x &= 5.29\times 10^{-6} (0.01\%) \end{align*}\]
Get pH.
\[\begin{align*} \mathrm{pOH} &= -\log[\mathrm{OH^-}] \\ &= -\log (5.29\times 10^{-6})\\ &= 5.28\\[2ex] \mathrm{pH} &= \mathrm{p}K_{\mathrm{w}} - \mathrm{pOH}\\ &= 14 - 5.28\\ &= 8.72 \end{align*}\]
Beyond the equivalence point
What is the pH after 30.0 mL of NaOH is added?
Solution
Step 1: Determine acid/base reaction type
- This is a weak acid/strong base titration
- \(\mathrm{HA}(aq) + \mathrm{OH^-}(aq) \rightleftharpoons \mathrm{H_2O}(l) + \mathrm{A^-}(aq)\)
Step 2: Determine molar changes (IRF table)
To determine molar changes, convert concentration (M) to moles
\[\begin{align*} n_{\mathrm{HA}} &= (0.0250~\mathrm{L})\left(0.100~\mathrm{mol~L}^{-1}\right)\\ &= 0.00250~\mathrm{mol~HA} \\[2ex] n_{\mathrm{OH}^{-}} &= (0.0300~\mathrm{L})\left(0.100~\mathrm{mol~L}^{-1}\right)\\ &= 0.00300~\mathrm{mol~OH}^{-} \end{align*}\]
React acid and base (in moles).
| Initial moles | 0.00250 | 0.00300 | 0 | ||||
| Reaction | HA | + | OH– | → | H2O | + | A– |
| Final moles | 0 | 0.0005 | 0.00250 |
Step 3: Determine final pH
A strong species (OH–) remains. Find the total volume of solution and convert moles back into molarity (M). Get pH.
\[\begin{align*} V_{\mathrm{final}} &= 25.0~\mathrm{mL} + 30.0~\mathrm{mL}\\ &= 55.0~\mathrm{mL}\\ &= 0.055~\mathrm{L}\\[2ex] [\mathrm{OH^-}] &= \dfrac{0.0005~\mathrm{mol}}{\mathrm{0.055~L}}\\[1.5ex] &= 0.0091~M \\[2ex] \mathrm{pOH} &= -\log [\mathrm{OH^-}] \\ &= -\log (0.0091) = 2.04 \\[2ex] \mathrm{pH} &= \mathrm{p}K_{\mathrm{w}} - \mathrm{pOH}\\ &= 14 - 2.04 \\ &= 11.96 \end{align*}\]
5.11.3 Practice
Below are tables for two different titrations (strong acid/strong base and weak acid/strong base). Try to replicate the pH in the last column by using only the data in column 1.
5.11.3.1 Strong acid/strong base titration
Titrate 25.0 mL of 0.100 M HCl with 0.100 M NaOH. (at 25 °C) What is the pH after X mL (from column 1) of NaOH is added?
| VOH– (mL) | nOH– (mol) | nH+ remain (mol) | Vtot (mL) | [H+] (M) | pOH | pH | |
|---|---|---|---|---|---|---|---|
0 |
0 |
0.0025 |
25 |
0.1 |
13.00 |
1.00 | |
5 |
0.0005 |
0.002 |
30 |
0.0667 |
12.82 |
1.18 | |
10 |
0.001 |
0.0015 |
35 |
0.0429 |
12.64 |
1.36 | |
15 |
0.0015 |
0.001 |
40 |
0.025 |
12.40 |
1.60 | |
20 |
0.002 |
0.0005 |
45 |
0.011 |
12.04 |
1.96 | |
25 |
0.0025 |
0 |
50 |
1.0 ×10–7 |
7.00 |
7.00 |
| VOH– (mL) | nOH– (mol) | Excess OH– (mol) | Vtot (mL) | [OH–] (M) | pOH | pH | |
|---|---|---|---|---|---|---|---|
30 |
0.003 |
0.0005 |
55 |
0.0091 |
2.04 |
11.96 | |
35 |
0.0035 |
0.001 |
60 |
0.0167 |
1.78 |
12.22 |
5.11.3.2 Weak acid/strong base titration
Titrate 25.0 mL of 0.100 M CH3COOH with 0.100 M NaOH (at 25 °C). What is the pH after X mL (from column 1) of NaOH is added? Ka(CH3COOH) = 1.80 ×10–5
| VOH– (mL) | nOH– (mol) | HA remain (mol) | A– made (mol) | Vtot (mL) | pOH | pH | |
|---|---|---|---|---|---|---|---|
0 |
0 |
0.0025 |
0 |
25 |
11.13 |
2.87 | |
5 |
0.0005 |
0.002 |
0.0005 |
30 |
9.86 |
4.14 | |
10 |
0.001 |
0.0015 |
0.001 |
35 |
9.44 |
4.56 | |
15 |
0.0015 |
0.001 |
0.0015 |
40 |
9.08 |
4.92 | |
20 |
0.002 |
0.0005 |
0.002 |
45 |
8.66 |
5.34 | |
25 |
0.0025 |
0 |
0.0025 |
50 |
5.28 |
8.72 |
| VOH– (mL) | nOH– (mol) | Excess OH– (mol) | Vtot (mL) | [OH–] (M) | pOH | pH | |
|---|---|---|---|---|---|---|---|
30 |
0.003 |
5e-04 |
55 |
0.0091 |
2.04 |
11.96 | |
35 |
0.0035 |
0.001 |
60 |
0.0167 |
1.778 |
12.22 |