1.4 Surface Tension
Surface tension is a property of a liquid that allows the surface of the liquid to resist an external force due to the cohesive forces (attractions) between the molecules.
A paperclip or an insect is able to float on the surface of water because the water molecules are strongly attracted to each other and the force applied to its surface is not strong enough to break the molecular interactions.
](files/ch10/insect-on-water.jpg)
Figure 1.14: An insect standing on the surface of water. Source
The stronger the intermolecular forces between the molecules, the larger the surface tension.
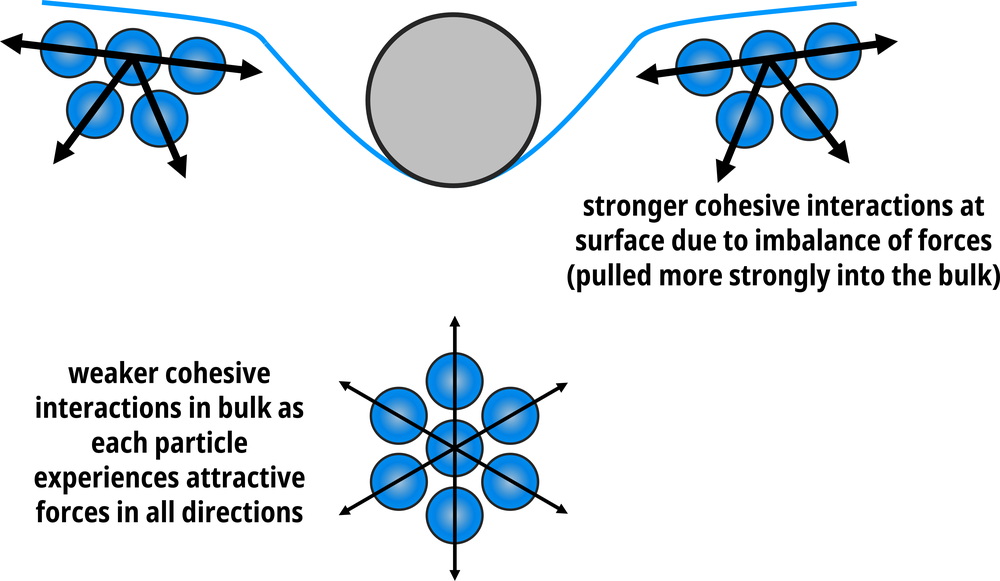
Figure 1.15: Surface molecules have stronger cohesive forces (attractions) than those in the bulk.
Surface tension is what allows liquids to bead (i.e. minimize their surface areas – this is why water forms into round droplets).
](files/ch10/water-alcohol-surface-tension.jpg)
Figure 1.16: Drops of water vs. rubbing alcohol on the surface of a penny. Source
Surface tension decreases with increasing temperature. As the particles in a liquid gain kinetic energy, the intermolecular forces are shorter-lived and therefore, weaker.
Figure 1.17: Surface tension of some substances vs. temperature
Given below are surface tensions of some substances at 25 °C.
Figure 1.18: Surface tension of some substances at 25 °C.