Chapter 3 Kinetics
Chemistry can be divided (somewhat arbitrarily) into the study of structures, equilibria, and rates. Chemical structure is ultimately described by the methods of quantum mechanics; equilibrium phenomena are studied by statistical mechanics and thermodynamics; and the study of rates constitutes the subject of kinetics.
Kinetics can be subdivided into physical kinetics, dealing with physical phenomena such as diffusion and viscosity, and chemical kinetics, which deals with the rates of chemical reactions (including both covalent and noncovalent bond changes).– Connors, K.8
Key concepts:
- Kinetics describes the rates of reactions
- Rates can be affected by reactant concentrations as well as environmental factors such as temperature
- Reaction profiles can be drawn as reaction pathways
Not all reactions are the same
Reactions occur at some “speed” or “rate”. A combustion reaction of gasoline and oxygen is very fast while the oxidation of iron (i.e. rust) is relatively slower. Reaction rates can be expressed in different ways. Imagine a reaction taking place in aqueous solution with a single reactant, A.
\[\mathrm{A}(aq) \longrightarrow \mathrm{products}\]
We can track the molar concentration of A vs. time (t). Here, the initial molar concentration of A is 1.0 M and the consumption of reactant A with respect to time has the form
\[y = e^{-x}\]
or
\[[\mathrm{A}] = e^{-t}\]
The plot below illustrates the consumption of reactant A with time.
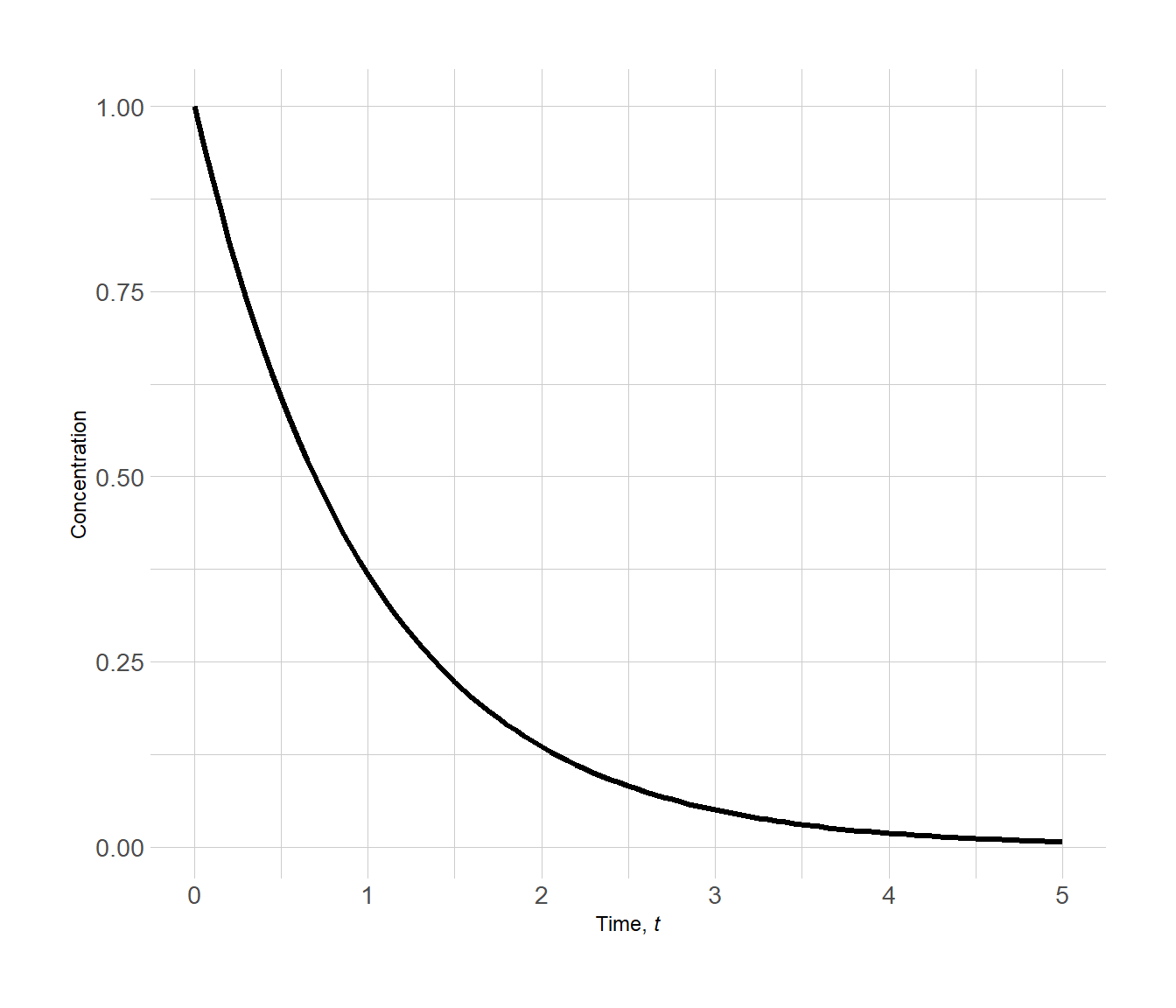
Figure 3.1: Concentration vs. time plot of a simple reaction involving reactant A in solution
This behavior can be expressed as an average rate in the form of
\[\dfrac{-\Delta [\mathrm{A}]}{\Delta t}\]
stated as the change in the concentration of A with respect to a change in time. By making t infinitesimally small, we converge on an infinitesimally small change in concentration giving an instantaneous (or differential) rate.
\[\dfrac{-\delta[\mathrm{A}]}{\delta t}\]
Given that reactant A is being consumed, a negative sign is included to keep the value positive.
All reaction rates are positive.
References
(8)Connors, K. A. Chemical Kinetics: The Study of Reaction Rates in Solution; VCH Publishers, 1990.

