7.2 Gibbs Energy
Gibbs free energy (or Gibbs energy; G) is a thermodynamic potential that is minimized when a chemical system reaches chemical equilibrium. The Gibbs free energy change for a reaction, ΔG, is used to define if a reaction or process is spontaneous or non-spontaneous.
| Sign | Feasibility | Favor |
|---|---|---|
| ΔG < 0 | feasible | product favored |
| ΔG > 0 | not feasible | reactant favored |
| ΔG = 0 | at equilibrium | none |
The Gibbs free energy equation determines the Gibbs energy for a reaction by taking into account the enthalpy, ΔH, and entropy, ΔS, of reaction.
\[\Delta G^{\circ} = \Delta H^{\circ} - T\Delta S^{\circ}\]
Note: The “degree” symbols indicate that the thermodynamic values were obtained for substances in their standard state (T = 25 °C, P = 1 bar, c = 1 M). Appendix G in Chemistry 2e reports these values for many substances. Also note that T in the Gibbs free energy equation can be any temperature, not simply 298.15 K.
Let us look up the thermodynamic values for the following reaction.
\[\mathrm{2NO_2}(g) \rightleftharpoons 2\mathrm{NO}(g) + \mathrm{O_2}(g)\]
All values in the table below are given in kJ mol–1.
| Species | ΔHf° | S° |
|---|---|---|
NO2(g) |
33.2 |
0.2401 |
NO(g) |
90.25 |
0.2108 |
O2 |
0 |
0.2052 |
We can determine the enthalpy of reaction by
\[\Delta H_{\mathrm{rxn}} = \Sigma \left (\Delta H_{\mathrm{products}} \right ) - \Sigma \left (\Delta H_{\mathrm{reactants}} \right )\] Therefore,
\[\begin{align*} \Delta H_{\mathrm{rxn}} &= \left [ (2 \times 90.25) + (0) \right ] - \left [ (2\times 33.2) \right ] \\[1.5ex] &= 114.1~\mathrm{kJ~mol^{-1}} \end{align*}\]
This reaction is endothermic! We repeat this process for the entropy of reaction.
\[\Delta S_{\mathrm{rxn}} = \Sigma \left (\Delta S_{\mathrm{products}} \right ) - \Sigma \left (\Delta S_{\mathrm{reactants}} \right )\] to give
\[\begin{align*} \Delta S_{\mathrm{rxn}} &= \left [ (2 \times 0.2108) + (0.2052) \right ] - \left [ (2\times 0.2401) \right ] \\[1.5ex] &= 0.1466~\mathrm{kJ~mol^{-1}} \end{align*}\]
The reaction experiences an increase in entropy. This is expected becuase we go from 2 moles of gas to 3 moles of gas!
Now we determine the Gibbs free energy for the reaction using the Gibbs free energy equation.
\[\begin{align*} \Delta G^{\circ} &= \Delta H^{\circ} - T\Delta S^{\circ} \\ &= 114.1~\mathrm{kJ~mol^{-1}} - T(0.1466~\mathrm{kJ~mol^{-1}}) \end{align*}\]
I tabulate the Gibbs free energy (in kJ mol–1 for this reaction at three different temperatures, T, in the table below.
| T (°C) | T (K) | ΔG° |
|---|---|---|
-200 |
73.15 |
103.38 |
25 |
298.15 |
70.39 |
600 |
873.15 |
-13.9 |
We see that at low temperatures, the Gibbs free energy is positive. The reaction is non-spontaneous at low temperatures. As we increase the temperature, the reaction eventually becomes spontaneous (see how ΔG < 0 at higher T).
According to Le Chatelier’s principle, increasing the heat for an endothermic reaction should drive the equilibrium to the right. We can determine the equilibrium constant for this reaction at the three temperatures of interest using
\[\Delta G^{\circ} = -RT\ln K\]
where R is the gas constant, T is the temperature, and K is the equilibrium constant. Rearranging for K gives
\[K = e^{\frac{-\Delta G^{\circ}}{RT}}\]
I’ve added the equilibrium constants in our table below.
| T (°C) | T (K) | ΔG° | K |
|---|---|---|---|
-200 |
73.15 |
103.38 |
1.51e-74 |
25 |
298.15 |
70.39 |
4.65e-13 |
600 |
873.15 |
-13.9 |
6.79 |
We see for this endothermic reaction that the equilibrium constant gets larger (favors product) as the temperature goes up! This fits with Le Chatelier’s principle! Also, note how ΔG is decreasing with increasing temperature for this reaction. At high temperatures, this reaction is spontaneous and products is favored. We note the correlation
\[\downarrow \Delta G \propto K \uparrow\]
7.2.1 Effect of Enthalpy and Entropy
The sign of ΔG from the Gibbs free energy equation is affected by the sign of ΔH and ΔS.
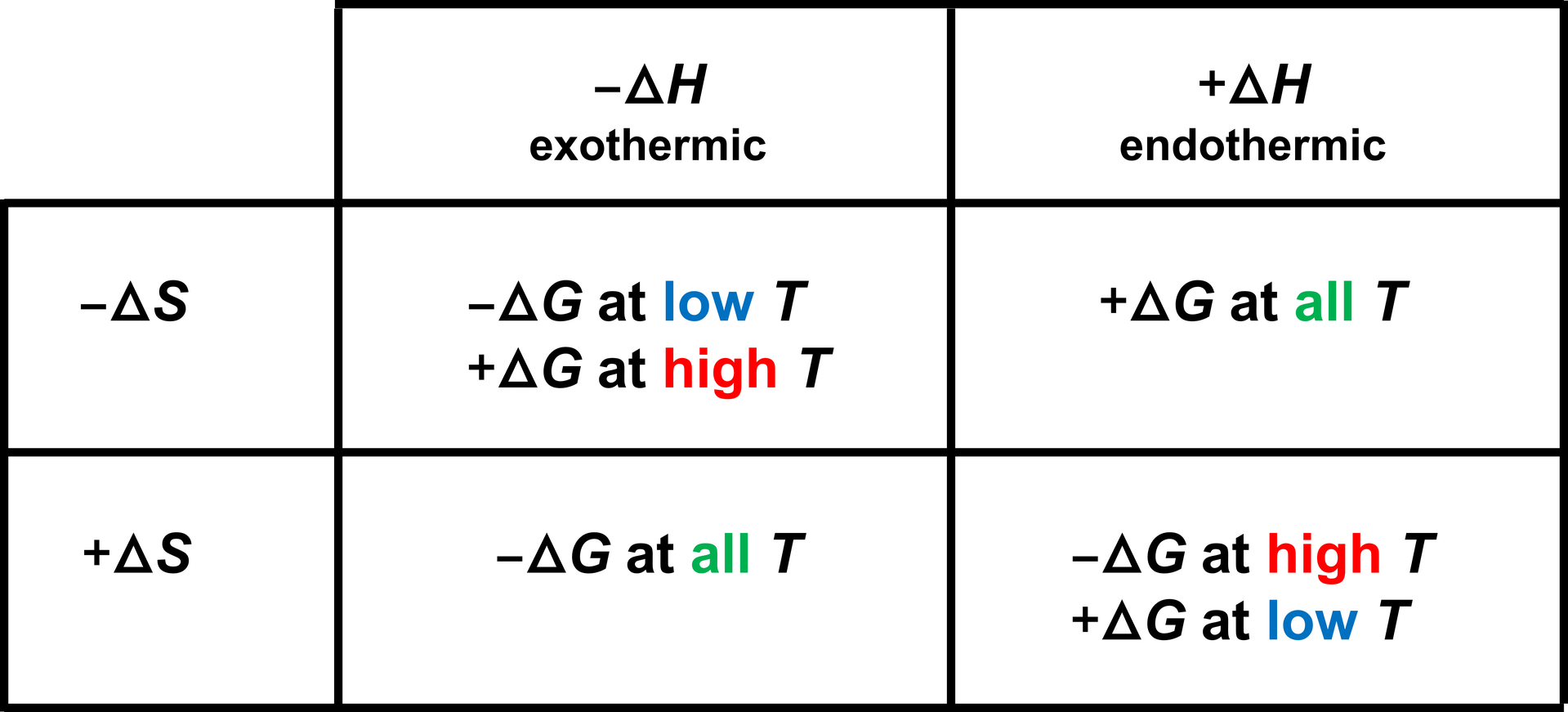
I outline the four cases below.
Case 1: ΔH < 0 and ΔS > 0
For any reaction that is exothermic (ΔH < 0) with an increase in entropy (ΔS > 0), the reaction is thermodynamically feasible (ΔG < 0) at all temperatures.
\[\begin{align*} \Delta G &= \Delta H - T\Delta S \\[1.25ex] &= (-) - T(+) \\[1.25ex] &< 0 \quad (\mathrm{at~any}~T) \end{align*}\]
Example: The burning of a fuel that generates a gas is always spontaneous. Heat is given off and the entropy increases.
Case 2: ΔH > 0 and ΔS < 0
For any reaction that is endothermic (ΔH > 0) with an decrease in entropy (ΔS < 0), the reaction is not thermodynamically feasible (ΔG > 0) at any temperature.
\[\begin{align*} \Delta G &= \Delta H - T\Delta S \\[1.25ex] &= (+) - T(-) \\[1.25ex] &= (+) + T(+) \\[1.25ex] &> 0 \quad (\mathrm{at~any}~T) \end{align*}\]
Example: The formation of diamonds from coal and photosynthesis are never spontaneous.
Case 3: ΔH < 0 and ΔS < 0
For any reaction that is exothermic (ΔH < 0) with a decrease in entropy (ΔS < 0), the reaction is thermodynamically feasible (ΔG < 0) at lower temperatures and not thermodynamically feasible at higher temperatures.
\[\begin{align*} \Delta G &= \Delta H - T\Delta S \\[1.25ex] &= (-) - T(-) \\[1.25ex] &= (-) + T(+) \\[1.25ex] &< 0 \quad (\mathrm{at~lower}~T) \end{align*}\]
Example: Water will spontaneously freeze at lower temperatures (T < 0 °C) but will not spontaneously freeze at higher temperatures (T > 0 °C).
Case 4: ΔH > 0 and ΔS > 0
For any reaction that is endothermic (ΔH > 0) with an increase in entropy (ΔS > 0), the reaction is thermodynamically feasible (ΔG < 0) at higher temperatures and not thermodynamically feasible at lower temperatures.
\[\begin{align*} \Delta G &= \Delta H - T\Delta S \\[1.25ex] &= (+) - T(+) \\[1.25ex] &< 0 \quad (\mathrm{at~higher}~T) \end{align*}\]
Example: Ice will spontaneously melt at higher temperatures (T > 0 °C) but will not spontaneously melt at lower temperatures (T < 0 °C).
7.2.2 Entropy vs Free Energy
- Entropy is energy that cannot be used to do work.
- Free energy is the energy able to do work.
- Entropy is energy that must be present for something to exist in the state it is in.
Consider the following combustion reaction of acetylene (C2H2):
\[\mathrm{C_2H_2}(g) + 2\mathrm{O_2}(g) \longrightarrow \mathrm{CO_2}(g) + 2\mathrm{H_2O}(g)\]
This reaction is very exothermic where the standard heat of reaction is –1192.57 kJ mol–1. Therefore, one might expect to be able to extract all this heat energy to do some other work. But, this is not the case. The entropy of this reaction is –0.2575 kJ mol–1 K–1.
We use the Gibbs free energy equation to determine the maximum amount of heat energy that can be extracted to do work from this process. If this reaction were carried out under standard conditions such as 25 °C, ΔG° is
\[\begin{align*} \Delta G^{\circ} &= \Delta H^{\circ} - T\Delta S^{\circ} \\[1.5ex] &= -1192.57~\mathrm{kJ~mol^{-1}} - (298.15~\mathrm{K})(-0.2575~\mathrm{kJ~mol^{-1}~K^{-1}})\\[1.5ex] &= -1115.8~\mathrm{kJ~mol^{-1}} \end{align*}\]
We see that the maximum available energy to do work (ΔG°) is less than the energy released by the reaction (ΔH°). Some of the released energy is retained by (distributed to) the surroundings. While the system experienced a decrease in entropy, the surroundings experienced an increase in entropy indicating that some of the released energy was distributed uniformly to the surroundings and cannot be used to do work anymore.
\[\begin{align*} \Delta S_{\mathrm{surr}} &= \dfrac{\Delta H}{T} \\[1.25ex] &= \dfrac{1192.57~\mathrm{kJ~mol^{-1}}}{298.15~\mathrm{K}} \\[1.25ex] &= 4~\mathrm{kJ~mol^{-1}~K^{-1}} \end{align*}\]
Entropy exists on an absolute scale. All matter possesses entropy and at 0 Kelvin, a perfect crystal of a perfect substance, entropy is defined to be zero. As temperature increases, entropy increases.