1.1 Intermolecular Forces
Intermolecular forces are attractive interactions between the molecules. These forces are responsible for keeping molecules in a liquid in close proximity with neighboring molecules.
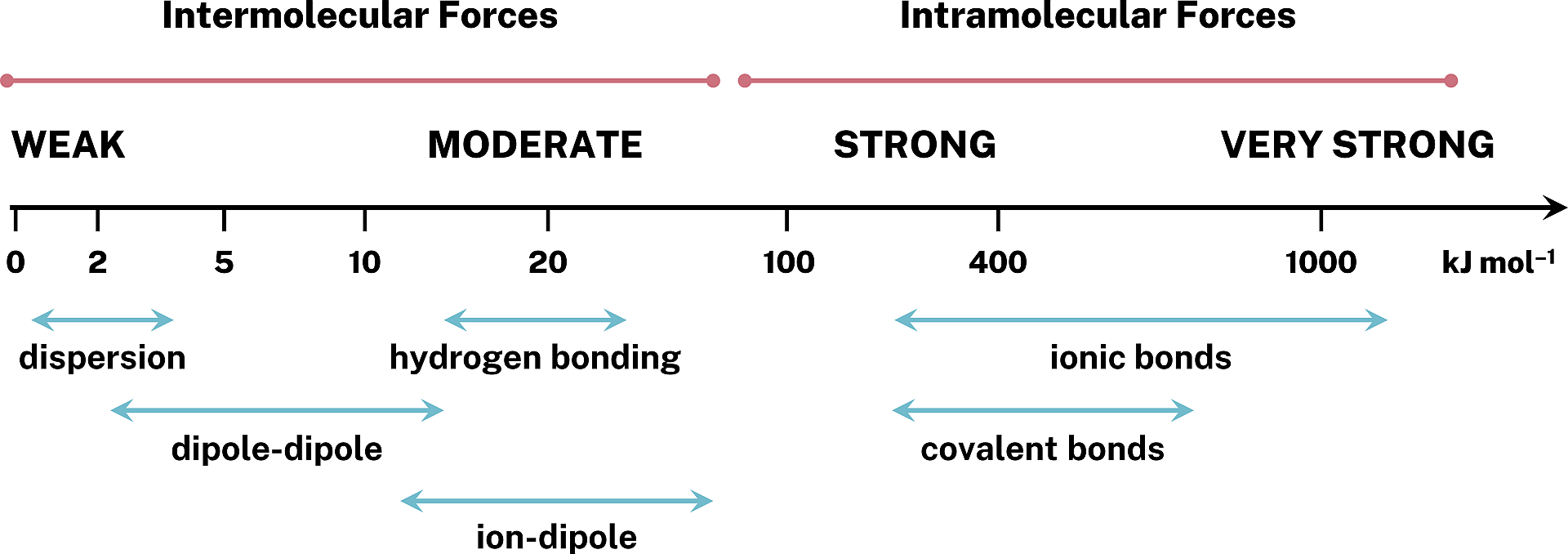
Figure 1.2: Relative strengths of some attractive intermolecular forces.
Dispersion is the weakest intermolecular force and is the dominant intermolecular force in non-polar molecules. It is important to note that molecules can exhibit multiple intermolecular forces. Dispersion exists in all substances.
Dipole-dipole interactions exist between molecules with dipoles. Hydrogen bonding is a special type of dipole-dipole interaction that exists in molecules that contain a hydrogen bound to a highly electronegative atom (N, O, or F). Let us analyze the boiling points of series of hydrides for the following main group elements to highlight the enhanced nature of hydrogen bonding.
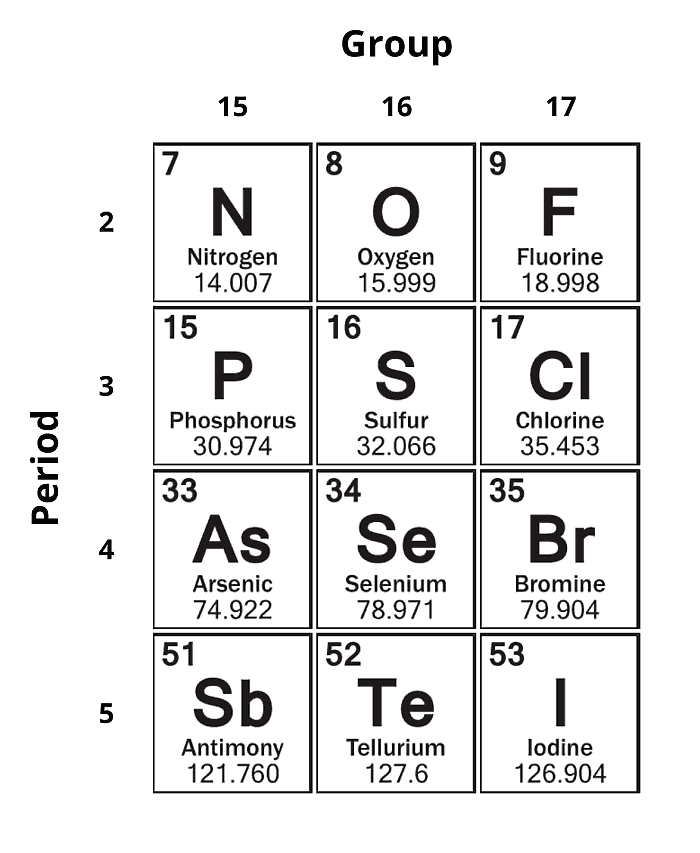
Figure 1.3: Hydrides of the following main group elements
Stronger intermolecular forces require more energy to break these interactions and generally lead to higher boiling points. These hydrides will be organized by Group (15, 16, and 17, respectively) moving down the periodic table. The boiling points are plotted by the period of the main group element.
Figure 1.4: Boiling points of some hydrides of main group elements
The data indicates that hydrogen bonding tend to be stronger than other dipole-dipole interactions.
The boiling point of some simple substances are given below and categorized by the dominant intermolecular force they exhibit. Note that ammonium chloride (NH4Cl), included for comparison, exhibits ionic intramolecular forces.
Figure 1.5: Boiling points of some molecules
It is clear that the types of intermolecular forces span a wide range of boiling points and, therefore, interaction energies. The classes of intermolecular forces overlap in terms of strength; however, a general trend is still seen. Dispersion tends to be the weakest intermolecular force while hydrogen-bonding, a special type of dipole-dipole interaction, tends to be strong.
Dispersion interactions are stronger in molecules with larger masses.
| Name | Formula | m.m. (g mol–1) | Boiling Pt. (°C) |
|---|---|---|---|
| Astatine | At2 |
420 | 610 |
| Iodine | I2 |
254 | 457 |
| Bromine | Br2 |
160 | 332 |
| Chlorine | Cl2 |
71 | 238 |
| Fluorine | F2 |
38 | 85 |
Dispersion also tends to be stronger in molecules with larger surface area.
| Name | Formula | m.m. (g mol–1) | Boiling Pt. (°C) |
|---|---|---|---|
n–pentane |
C5H12 |
72.15 | 36.0 |
isopentane |
C5H12 |
72.15 | 27.0 |
neopentane |
C5H12 |
72.15 | 9.5 |
Pentane
Isopentane
Neopentane
| Name | Formula | m.m. (g mol–1) | Boiling Pt. (°C) |
|---|---|---|---|
n–hexane |
C6H14 |
86.18 | 69.0 |
isohexane |
C6H14 |
86.18 | 60.0 |
neohexane |
C6H14 |
86.18 | 49.8 |
Hexane
Isohexane
2-Methylpentane
Neohexane
2,2-Dimethylbutane
Explain the following boiling points (below) using intermolecular forces.
| Name | Formula | Dominant IMF | Dipole | BP |
|---|---|---|---|---|
| Sodium chloride | NaCl |
Ion-Ion | 9.00 | 1465.00 |
| Ethylene glycol | (CH2OH)2 |
Hydrogen bonding | 2.75 | 197.60 |
| Pentanol | C5H11OH |
Hydrogen bonding | 1.70 | 138.00 |
| Benzene | C6H6 |
Dispersion | 0.00 | 80.08 |
| Methanol | CH3OH |
Hydrogen bonding | 1.69 | 64.70 |
| Pentane | C5H12 |
Dispersion | 0.00 | 36.06 |
| Neopentane | C5H12 |
Dispersion | 0.00 | 9.50 |
| Ethane | C2H6 |
Dispersion | 0.00 | -88.60 |
| Methane | CH4 |
Dispersion | 0.00 | -161.50 |
Ethylene glycol
Pentanol
Benzene
Methanol
Pentane
Neopentane
Ethane
Methane
CH4
Practice
Determine the dominant intermolecular force for the following molecules and rank the boiling points from smallest to largest.
- water (H2O)
- butane (C4H10)
- formaldehyde (H2CO)
- methane (CH4)
- methanol (CH3OH)
Solution
Dominant IMFs:
- water – hydrogen bonding
- butane (C4H10) – dispersion
- formaldehyde (H2CO) – dipole-dipole
- methane (CH4) – dispersion
- methanol (CH3OH) – hydrogen bonding
Boiling points:
methane < butane < formaldehyde < methanol < water
The intermolecular forces between particles give rise to the properties of different liquids.
Hydrogen-bonding in DNA
Adenine-Thymine Base Pair
The adenine and thymine nucleic acids interact via how many hydrogen bonds (in the given orientation)? Hydrogen atoms are white.