5.4 pH and pOH
The pH of an aqueous solution is a measure of the hydronium ion concentration.
\[\mathrm{pH} = -\log [\mathrm{H_3O^+}]\] and
\[[\mathrm{H_3O}^+] = 10^{-\mathrm{pH}}\]
The pOH is a measure of the hydroxide ion concentration
\[\mathrm{pOH} = -\log [\mathrm{OH^-}]\] and
\[[\mathrm{OH}^-] = 10^{-\mathrm{pOH}}\]
The pH and pOH of solution is related through pKw such that
\[\mathrm{p}K_{\mathrm{w}} = \mathrm{pH} + \mathrm{pOH}\]
Acidic, basic, and neutral solutions are defined by the relative amounts of H3O+ and OH– in the solution.
| Solution | at 25 °C | |
|---|---|---|
Acidic |
[H3O+] > [OH–] |
pH < 7, pOH > 7 |
Basic |
[H3O+] < [OH–] |
pH > 7, pOH < 7 |
Neutral |
[H3O+] = [OH–] |
pH = 7, pOH = 7 |
The image below illustrates a wide variety of substances across the pH scale of 0 to 14.
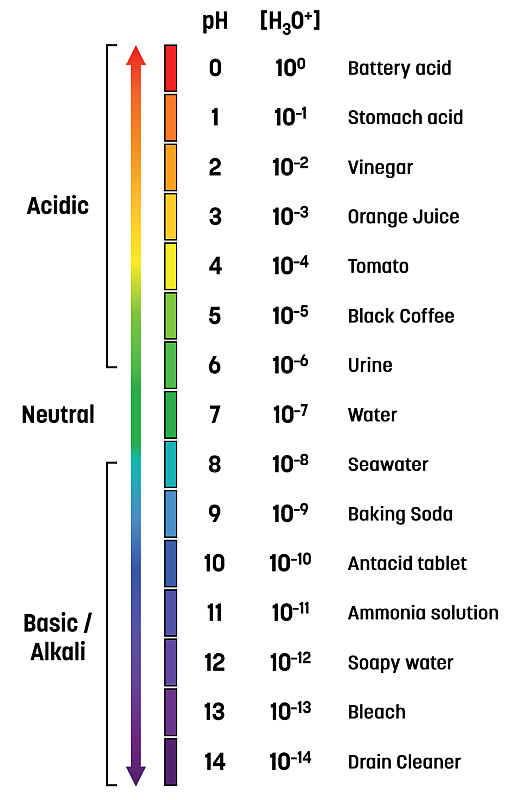
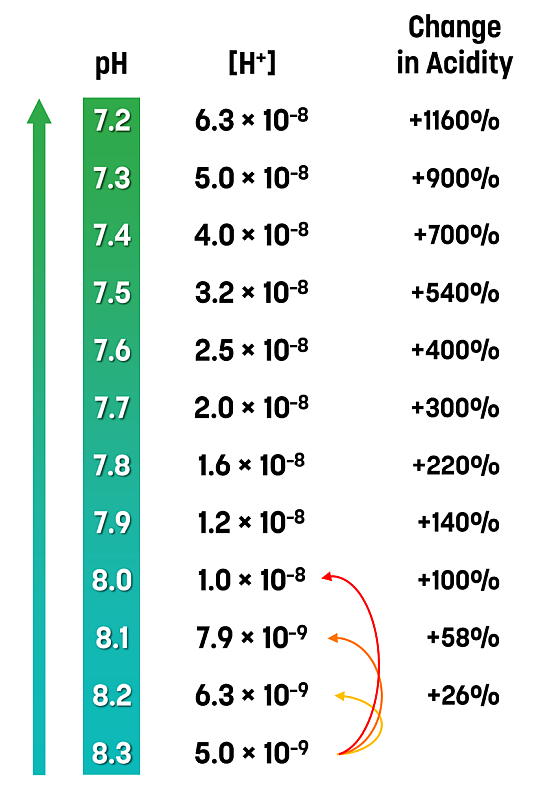
Given that pH is a log scale of hydronium ion concentration, it is important to understand that a small change in pH results in a large change in acidity.
Practice
The pH of an aqueous acidic solution is 3.45 at 25 °C. What is the pOH?
Solution
The pKw of water is 14 at 25 °C. Therefore,
\[\begin{align*} \mathrm{p}K_{\mathrm{w}} &= \mathrm{pH} + \mathrm{pOH} \\[1.5ex] \mathrm{pOH} &= \mathrm{p}K_{\mathrm{w}} - \mathrm{pH} \\[1.5ex] &= 14.00 - 3.45 \\[1.5ex] &= 10.55 \end{align*}\]
Practice
The pH of an aqueous acidic solution is 3.45 at 50 °C. What is the pOH?
Solution
The pKw of water is 13.26 at 50 °C. Therefore,
\[\begin{align*} \mathrm{p}K_{\mathrm{w}} &= \mathrm{pH} + \mathrm{pOH} \\[1.5ex] \mathrm{pOH} &= \mathrm{p}K_{\mathrm{w}} - \mathrm{pH} \\[1.5ex] &= 13.26 - 3.45 \\[1.5ex] &= 9.81 \end{align*}\]
Practice
The pH of pure water at 50 °C is 6.63. Is the water acidic, basic, or neutral?
Solution
Neutral. pH is equal to pOH.