8.4 Movement of Charge
Electricity is caused by the movement of charge such as the movement of electrons or ions. Some useful definitions are given below.
| Property | Definition | Measure or Unit |
|---|---|---|
Electric charge |
Charge on a proton |
1.602×10–19 C |
Electric current |
Movement of charge |
ampere = A = 1 C s–1 |
Electric potential |
Force trying to move the charge |
volt = V = J C–1 |
Faraday’s Constant |
Charge per mole of elementary charges |
96,485 C mol–1 |
Charge is given in Coulombs (C; with SI units of A·s) and is defined as the charge on a proton, an elementary charge. Current is the movement of charge and is called amps (A) given as C s–1. The ampere is 1 Coulomb of charge (equivalent to 6.24151 × 1018 electrons) moving through a particular point in 1 second. The force moving the charge, called electric potential, is called volts (V) given as J C–1 (the energy per unit charge). Voltage is commonly referred to as electromotive force (emf).
8.4.1 Water Analogy
It can be useful to think of electricity in an analogous way such as the flow of water.
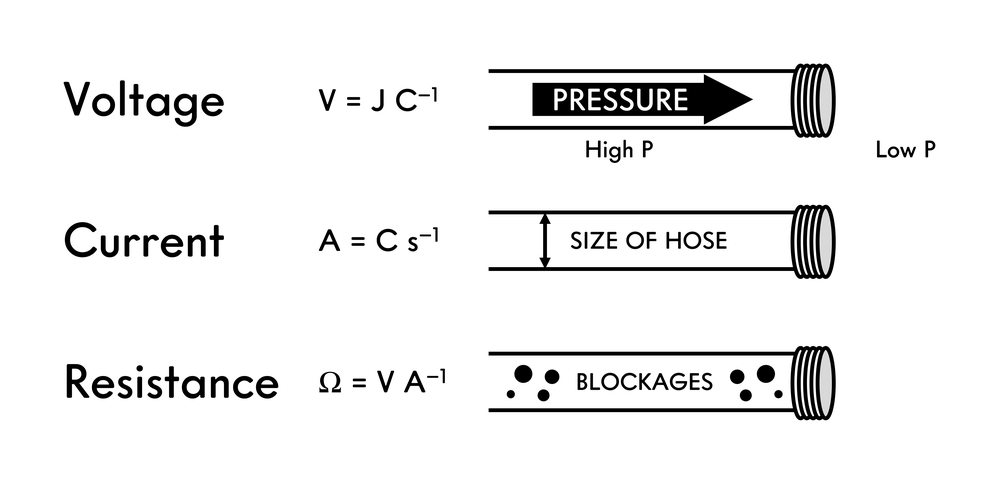
Voltage is analogous to the pressure difference (i.e. potential) that water experiences. Inside the hose, the pressure is high. At the exit of the hose, the pressure is low. The larger the difference in pressure, the more impressive the stream of water exiting the hose. We can see the effect of the force pushing a specified amount of water out of the hose. A higher the voltage, the larger the difference in the pressures, the more force is behind the movement of one Coulomb of charge. If the pressure inside the hose was equal to the pressure outside the hose, the water would not flow. However, there is still a force pushing the water. Therefore, charge can experience a large force pushing it (high voltage) but the charge may not flow due to an equally and opposite force pushing back on the charge (resistance, equilibrium, etc.). This would result in zero current (zero charge is moving). A battery that is not hooked up to a circuit is an example of a system having a voltage (i.e. an electric potential) without any current (without any movement of charge).
Current is analogous to the flow of water which correlates to the size of the hose that water passes through. If a hose is large, more water can flow through a point in the hose than a small hose would allow. A large current means more charge is flowing (per unit time). Low current means less charge is flowing per unit time. A battery in an open circuit will experience a current (flow of charge).
When a battery “dies”, the electric potential is zero resulting in zero voltage and therefore, no current (charge flow stops). The potential at the anode and the cathode are equal and the difference is zero.
8.4.2 Faraday’s Constant
Faraday’s constant is the amount of charge (in C) per mole of elementary charges and is a positive quantity. For example, the elementary charge of a proton is 1.602 × 10–19 C. A mole of protons would have a charge of 96,485 C. Similarly, the charge of an electron is equal but opposite in magnitude of a proton (-1.602 × 10–19 C). Therefore, the charge per mole of electrons (as a positive value) is 1.602 × 10–19 C.
Voltage is related to potential energy per unit charge such that
\[\mathrm{V} = \dfrac{\mathrm{potential~energy}}{\mathrm{charge}} = \dfrac{\mathrm{J}}{\mathrm{C}}\]
since volts is the force (energy) trying to move a charge. Another way, a charge of one coulomb that experiences an energy difference of 1 Joule between two potentials (e.g. between two electrodes), means the potential difference is exactly 1 volt. If a charge of one coulomb experiences an energy difference of 2 Joules, the potential difference is 2 volts. If a charge of two coulombs experiences an energy difference of 1 Joule, the potential difference is 0.5 volts.
Charge is therefore related to energy such that
\[\mathrm{C} = \dfrac{\mathrm{potenial~energy}}{\mathrm{electric~potential}} = \dfrac{\mathrm{J}}{\mathrm{V}}\]
A higher potential energy or lower electric potential leads to more charge.
The Joule (J; unit of energy), is therefore the work done to move an electric charge (C) through an electric potential of one volt (V).
\[1~\mathrm{J} = 1~\mathrm{C~V}\] The more charge there is, or the larger the electric potential, the more work that can be performed. Batteries that generate more voltage have a larger electric potential between the electrodes and/or more charge that can be transferred.
Faraday’s constant can be expressed in units of kJ.
\[F = \dfrac{96,485~\mathrm{C}}{\mathrm{mol}} = \dfrac{96,485~\mathrm{J}}{\mathrm{mol~V}} = \dfrac{96.485~\mathrm{kJ}}{\mathrm{mol~V}}\]