3.1 Average Rate
An average rate of reaction is simply a change in reactant (or product) concentration over an amount of time. We can choose two points in time (t1 and t2) and measure the concentration of the reactant at both of those points (c1 and c2). The average rate of reaction for that time frame via the following expression
\[\mathrm{avg.~rate} = -\dfrac{\Delta c}{\Delta t} = -\dfrac{c_2 - c_1}{t_2-t_1} \] Notice the negative sign. Since the concentration of the reactant is decreasing with increasing time, the numerator will be negative; but, rates are positive so we insert a negative sign to ensure a positive value.
For our reaction, let us choose two points.
| Point | Time | Concentration |
|---|---|---|
| 1 | 0.25 | 0.7788 |
| 2 | 2.25 | 0.1050 |
The plot below shows the two points (in blue).
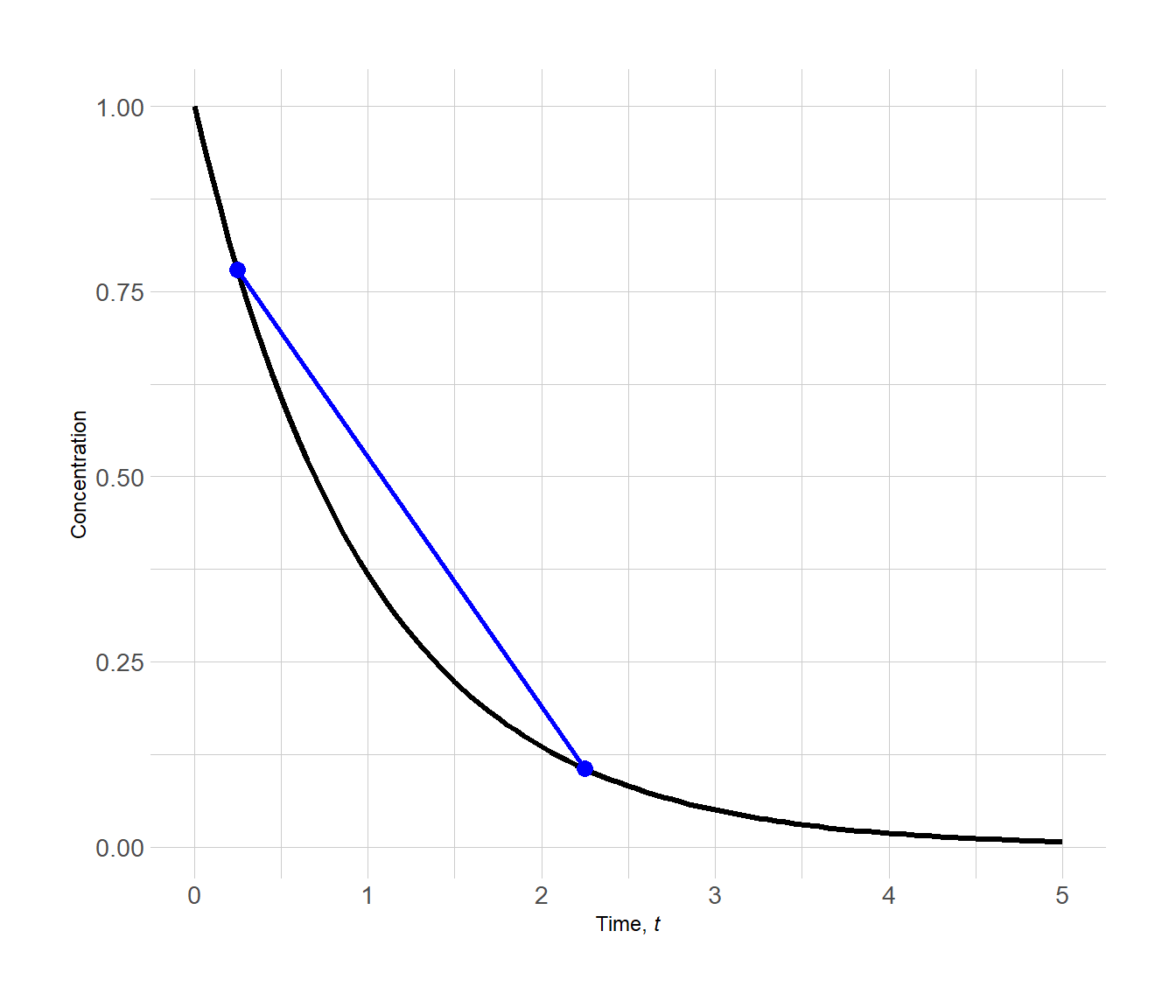
Figure 3.2: Determining the average rate of reaction by determining the slope a line between two points on a concentration vs. time plot
The slope of the line connecting the two points gives the average rate of reaction across the chosen time interval 0.25 → 2.25.
\[\begin{align*} \mathrm{avg.~rate} &= -\dfrac{c_2 - c_1}{t_2-t_1} \\[1.5ex] &= -\dfrac{0.1050 - 0.7788}{2.25 - 0.25} \\[1.5ex] &= 0.3369~c~t^{-1} \end{align*}\]
where c is simply the unit of concentration and t is a unit of time.
Let us choose another two points in the reaction and recalculate the average rate.
| Point | Time | Concentration |
|---|---|---|
| 3 | 2 | 0.1353 |
| 4 | 3 | 0.0183 |
Notice the shallow slope of the line. The average rate of the reaction has slowed down as the reaction progressed (i.e. as reactant A is consumed).
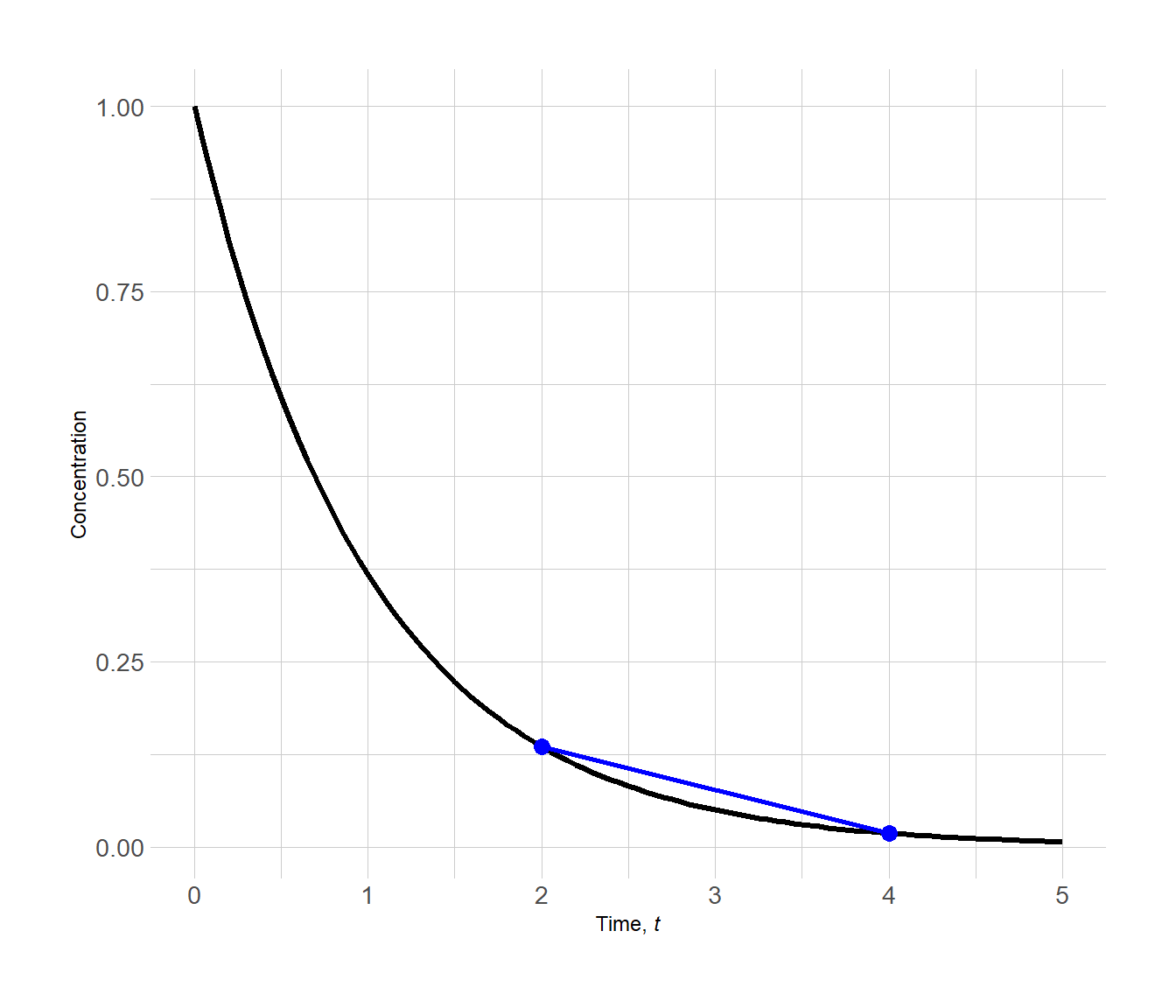
Figure 3.3: Determining the average rate of reaction by determining the slope a line between two points on a concentration vs. time plot
We calculate the average rate of reaction here for the time interval 2.00 → 4.00.
\[\begin{align*} \mathrm{avg.~rate} &= -\dfrac{c_2 - c_1}{t_2-t_1} \\[1.5ex] &= -\dfrac{0.0183 - 0.1353}{4.00 - 2.00} \\[1.5ex] &= 0.0585~c~t^{-1} \end{align*}\]
It is important to realize that the rate of a reaction can change as the reaction progresses!
Note: We could track the appearance of product and determine the average rate of reaction. Because the change in product concentration is positive, we would not include the negative sign as our rate will already be positive.
3.1.1 General Expression
The average rate of reaction can be expressed from a balanced chemical equation. We must take into account the stoichiometric coefficients.
For a general reaction
\[a\mathrm{A} + b\mathrm{B} \longrightarrow c\mathrm{C} + d\mathrm{D}\]
the average rate of reaction is written (with molar concentrations) as follows
\[ \require{color} \mathrm{avg.~rate} = \dfrac{1}{a}\textcolor{red}{\dfrac{-\Delta [\mathrm{A}]}{\Delta t}} = \dfrac{1}{b}\textcolor{blue}{\dfrac{-\Delta [\mathrm{B}]}{\Delta t}} = \dfrac{1}{c}\textcolor{green}{\dfrac{\Delta [\mathrm{C}]}{\Delta t}} = \dfrac{1}{d}\textcolor{orange}{\dfrac{\Delta [\mathrm{D}]}{\Delta t}} \]
The fractions that are colored are as follows:
- red - “rate of consumption of A” = a positive number
- blue - “rate of consumption of B” = a positive number
- green - “rate of production of C” = a positive number
- orange - “rate of production of D” = a positive number
Similar words for “consumption” (e.g disappearance, expenditure, depletion, etc.) and “production” (e.g. appearance, creation, etc.) can be used.
It is critical to rationalize these terms. They are all rates. Rates are numbers. The fraction, when evaluated, equals a rate which is simply a number. When these rates (given in color) are multiplied by one over the stoichiometric coefficient (from the balanced chemical equation), you obtain the average rate of reaction!
Practice
What is the average rate expression for the following reaction?
\[2\mathrm{NO}_2(g) \longrightarrow \mathrm{N_2O_4}(g)\]
Solution
\[\mathrm{avg.~rate} = \dfrac{1}{2}\dfrac{-\Delta[\mathrm{NO_2}]}{\Delta t} = \dfrac{\Delta[\mathrm{N_2O_4}]}{\Delta t}\]
Let’s see if we understand what the fractions mean.
Practice
What is the average rate of reaction (in M s–1) if the rate of disappearance of NO2 = 0.125 M s–1?
\[2\mathrm{NO}_2(g) \longrightarrow \mathrm{N_2O_4}(g)\]
Solution
\[\begin{align*} \mathrm{avg.~rate} &= \dfrac{1}{2}\dfrac{-\Delta[\mathrm{NO_2}]}{\Delta t} = \dfrac{\Delta[\mathrm{N_2O_4}]}{\Delta t}\\[5ex] \mathrm{avg.~rate} &= \dfrac{1}{2}\dfrac{-\Delta[\mathrm{NO_2}]}{\Delta t} \\[1.5ex] &= \dfrac{1}{2} \left ( 0.125~M~\mathrm{s^{-1}} \right ) \\[1.5ex] &= 0.0625~M~\mathrm{s^{-1}} \end{align*}\]
Let’s try that again!
Practice
What is the average rate of reaction (in M s–1) if the rate of appearance of N2O4 = 0.250 M s–1?
\[2\mathrm{NO}_2(g) \longrightarrow \mathrm{N_2O_4}(g)\]
Solution
\[\begin{align*} \mathrm{avg.~rate} &= \dfrac{1}{2}\dfrac{-\Delta[\mathrm{NO_2}]}{\Delta t} = \dfrac{\Delta[\mathrm{N_2O_4}]}{\Delta t}\\[5ex] \mathrm{avg.~rate} &= \dfrac{\Delta[\mathrm{N_2O_4}]}{\Delta t} \\[1.5ex] &= 0.250~M~\mathrm{s^{-1}} \end{align*}\]
Once more.
Practice
What is the rate of disappearance of NO2 (in M s–1) if the rate of appearance of N2O4 = 0.300 M s–1?
\[2\mathrm{NO}_2(g) \longrightarrow \mathrm{N_2O_4}(g)\]
Solution
\[\begin{align*} \mathrm{avg.~rate} &= \dfrac{1}{2}\dfrac{-\Delta[\mathrm{NO_2}]}{\Delta t} = \dfrac{\Delta[\mathrm{N_2O_4}]}{\Delta t}\\[5ex] \dfrac{1}{2}\dfrac{-\Delta[\mathrm{NO_2}]}{\Delta t} &= \dfrac{\Delta[\mathrm{N_2O_4}]}{\Delta t} \\[1.5ex] \dfrac{-\Delta[\mathrm{NO_2}]}{\Delta t} &= 2 \left ( \dfrac{\Delta[\mathrm{N_2O_4}]}{\Delta t} \right) \\[1.5ex] &= 2\left ( 0.300~M~\mathrm{s^{-1}} \right )\\[1.5ex] &= 0.600~M~\mathrm{s^{-1}} \end{align*}\]
Practice
Fill in the table for the following reaction:
\[\mathrm{H}_{2} \mathrm{O}_{2}(a q) \longrightarrow \mathrm{H}_{2} \mathrm{O}(l)+\dfrac{1}{2}\mathrm{O}_{2}(g)\]
| Time (h) | $$\dfrac{-\Delta [\mathrm{H_2O_2}]}{\Delta t}$$ | $$\dfrac{\Delta [\mathrm{H_2O}]}{\Delta t}$$ | $$\dfrac{\Delta [\mathrm{O_2}]}{\Delta t}$$ | Rate |
|---|---|---|---|---|
0.00 |
… |
… |
… |
… |
6.00 |
0.0833 |
0.0833 |
0.04165 |
|
12.00 |
0.0417 |
0.0417 |
||
18.00 |
0.0208 |
|||
24.00 |
0.0103 |
Solution
| Time (h) | $$\dfrac{-\Delta [\mathrm{H_2O_2}]}{\Delta t}$$ | $$\dfrac{\Delta [\mathrm{H_2O}]}{\Delta t}$$ | $$\dfrac{\Delta [\mathrm{O_2}]}{\Delta t}$$ | Rate |
|---|---|---|---|---|
0.00 |
… |
… |
… |
… |
6.00 |
0.0833 |
0.0833 |
0.04165 |
0.0833 |
12.00 |
0.0417 |
0.0417 |
0.02085 |
0.0417 |
18.00 |
0.0208 |
0.0208 |
0.0104 |
0.0208 |
24.00 |
0.0103 |
0.0103 |
0.00515 |
0.0103 |