1.9 Solids
Liquids can be cooled to form a solid, a state of matter where the particles are generally fixed into a particular position. Solids can be crystalline where the particles are arranged in a definite, repeatable, and orderly pattern or amorphous where the particles lack long-range order (i.e. has no repeating patterns) as seen with glass, rubber, many polymers, pitch, and more.
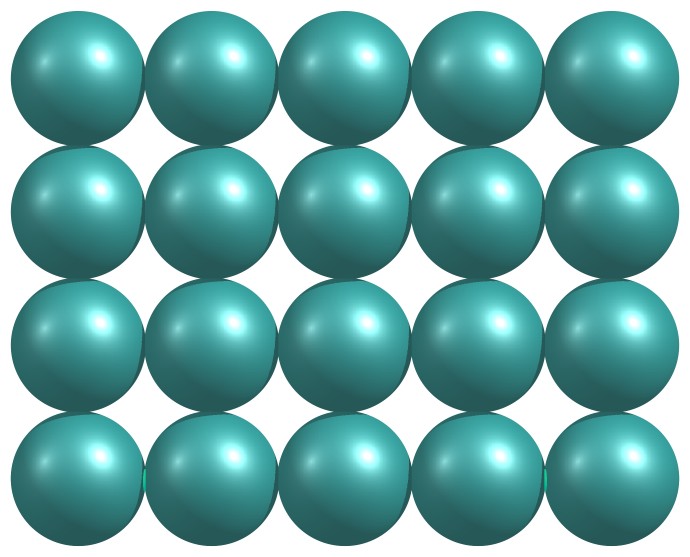
Figure 1.34: Ordered arrangement of particles in a crystalline solid.
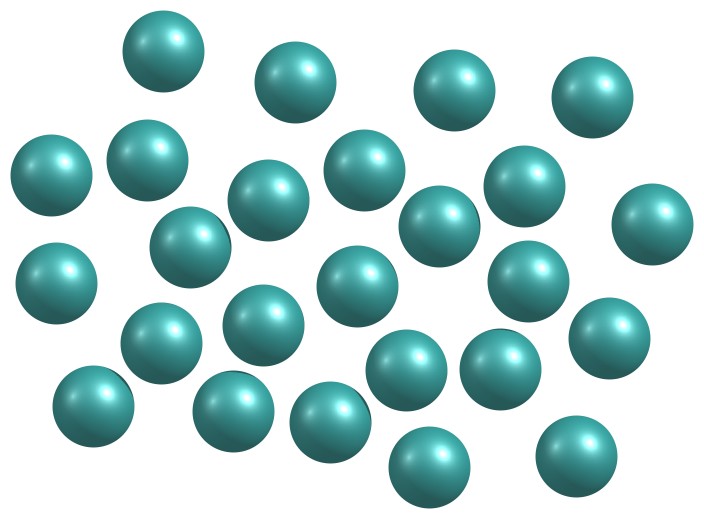
Figure 1.35: Disordered arrangement of particles in an amorphous solid.
Solids can be characterized into various types, each exhibiting a set of properties.
| Type | Particles | Attractions | Properties | Examples |
|---|---|---|---|---|
| ionic | ions | ionic | hard, brittle, conducts electricity as a liquid but not as a solid, high to very high melting points | NaCl, Al2O3 |
| metallic | atoms of electropositive elements | metallic | shiny, malleable, ductile, conducts heat and electricity well, variable hardness and melting points | Cu, Fe, Ti, Pb, U |
| covalent network | atoms of electronegative elements | covalent | very hard, not conductive, very high melting points | C (diamond), SiO2, SiC |
| molecular | molecules (or atoms) | IMFs | variable hardness, variable brittleness, not conductive, low melting points | H2O, CO2, I2, C12H22O11 |
1.9.1 Unit Cells
A unit cell is the most basic, repeatable pattern in a solid. There are many types of unit cells. Here we explore three types of cubic unit cells. These unit cells adopt the shape of a cube.
Simple cubic
A simple cubic (or primitive cubic) contains atoms located on the corners of a cube.
Body-centered cubic
A body-centered cubic contains atoms located on the corners of a cube as well as an atom located inside the cube at the center.
Face-centered cubic
A face-centered cubic contains atoms located on the corners of a cube as well as on the faces of the cube.
1.9.2 Equivalent Atoms
One metric for characterizing a unit cell is denoting the number of equivalent atoms in the unit cell. That is, equivalent atoms are the total number of atoms located inside the unit cell (i.e. the cube for cubic unit cells). For example, in our picture of the simple cubic unit cell, we see a total of eight spheres. However, only a fraction of each sphere actually resides inside the cube. Equivalent atoms only accounts for the total number of sphere fractions that are actually located inside the cube. Geometry tells us that a sphere that is centered on the corner of a cube will result in exactly 1/8th of that sphere lying inside the cube. Given that there are eight spheres, each located on a corner, we determine the number of equivalent atoms by totaling up the fractions of each sphere represented in the unit cell.
\[\mathrm{eq.~atoms} = \dfrac{1}{8} ~\left ( \mathrm{8~corner~spheres} \right ) = 1\] The fraction changes depending on the position of the sphere (shown below) and are as follows:
- Corner: 1/8
- Face: 1/2
- Edge: 1/4
A body-centered cubic contains 4 equivalent atoms.
\[\begin{align*} \mathrm{eq.~atoms} &= \dfrac{1}{8} ~\left ( \mathrm{8~corner~spheres} \right ) + 1 ~\left ( \mathrm{1~sphere~inside} \right ) \\ &= 2 \end{align*}\]
A face-centered cubic contains 4 equivalent atoms.
\[\begin{align*} \mathrm{eq.~atoms} &= \dfrac{1}{8} ~\left ( \mathrm{8~corner~spheres} \right ) + \dfrac{1}{2} ~\left ( \mathrm{6~faces~spheres} \right ) \\ &= 4 \end{align*}\]
The table below summarizes some characteristics of these cubic unit cells.
| Type | Equivalent Atoms | Structure | Coordination Number | Packing Efficiency |
|---|---|---|---|---|
| Simple cubic | 1 | 6 | 52% | |
| Body-centered cubic | 2 | 8 | 68% | |
| Face-centered cubic | 4 | 12 | 74% |
Note that the packing efficiency is a measure of the volume of the cube that is occupied by a sphere. For example, the cube of a primitive cubic has 52% of its volume occupied by spheres located at the lattice points, whereas, the body-centered cubic has an increased packing efficiency of 68% due to the additional sphere located at the center of the cube.
1.9.3 Empirical Formula
The number of equivalent atoms in a unit cell is directly related to the empirical formula for the substance. Consider the unit cell for cesium chloride (CsCl). Here, cesium is in purple and chlorine in green.
First, we recognize that this is a body-centered cubic. Therefore, there is exactly 1 equivalent Cs2+ atom (located in the center of the cube) and 1 equivalent Cl– atom (8 chlorides located on the corners of the cube). Therefore, we write the formula
\[\mathrm{Cs_1Cl_1} \longrightarrow \mathrm{CsCl}\]
and reduce the subscripts down to the lowest common denominator. What remains is the empirical formula for cesium chloride.
Practice
Determine the empirical formula for substance represented with the following unit cell. The purple spheres represent sodium and the green spheres represent chlorine.
Solution
Identify atoms and positions
- 13 purple atoms (sodium): 12 on edges and 1 in the center
\[\begin{align*} \mathrm{eq.~Na~atoms} &= \dfrac{1}{4} ~\left ( \mathrm{12~edge~spheres} \right ) + 1 ~\left ( \mathrm{1~sphere~inside} \right ) \\ &= 4 \end{align*}\]
- 14 green atoms (chlorine): 8 on corners and 6 on edges
\[\begin{align*} \mathrm{eq.~Cl~atoms} &= \dfrac{1}{8} ~\left ( \mathrm{8~corner~spheres} \right ) + \dfrac{1}{2} ~\left ( \mathrm{6~faces~spheres} \right ) \\ &= 4 \end{align*}\]
Determine empirical formula
\[\mathrm{Na_4Cl_4} \longrightarrow \mathrm{NaCl}\]
Practice
Determine the empirical formula for substance represented with the following unit cell. The gray spheres represent calcium and the brown spheres represent fluorine.
Solution
Identify atoms and positions
- 14 gray atoms (calcium): 8 on corners and 6 on edges
\[\begin{align*} \mathrm{eq.~Ca~atoms} &= \dfrac{1}{8} ~\left ( \mathrm{8~corner~spheres} \right ) + \dfrac{1}{2} ~\left ( \mathrm{6~faces~spheres} \right ) \\ &= 4 \end{align*}\]
- 8 brown atoms (fluorine): 8 inside the cube (tetrahedral holes)
\[\begin{align*} \mathrm{eq.~F~atoms} &= 8 \end{align*}\]
Determine empirical formula
\[\mathrm{Ca_4F_8} \longrightarrow \mathrm{CaF_2}\]